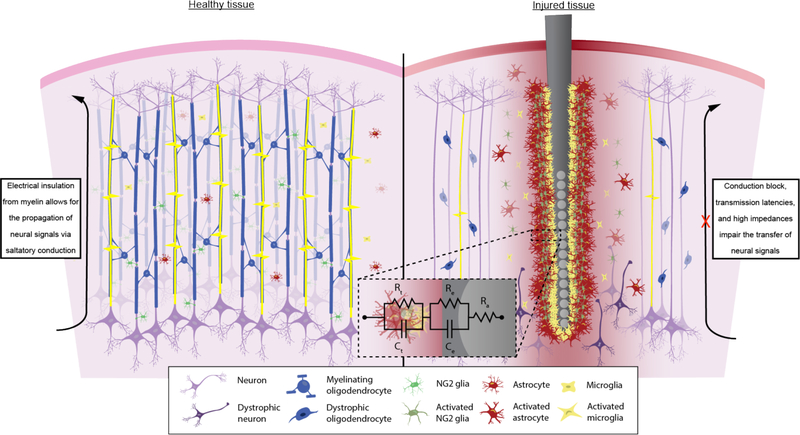
Figure 5. Schematic representation of the physiological consequences of microelectrode implantation in the brain.
The presence of the microelectrode has the potential to alter critical cellular functions that help maintain normal tissue homeostasis. NG2 glia are responsible for supporting neuronal health and modulating neuronal activity while maintaining a reservoir of oligodendrocyte precursors in the event of demyelination injury. Oligodendrocytes also support neuronal viability and produce insulating myelin sheaths that are necessary for signal propagation between neurons throughout the brain. Following electrode insertion, NG2 glia, along with microglia and astrocytes, become activated, proliferating and migrating toward the surface of the device. NG2 glia maintain the potential to differentiate into reactive astrocytes and participate in the encapsulation of the electrode via a glial scar. Scarring, which increases electrical impedances within the tissue (resistance and capacitance of the tissue, Rt and Ct), coupled with the impedance formed by the electrode-electrolyte layer (Re and Ce) as well as the electrode access resistance (Ra) impair ion exchange and reduce transfer of signal. Oligodendrocytes, which are easily susceptible to oxidative and excitotoxic injuries, succumb to inflammation and perish. The myelin structures they established begin to degrade leaving axons demyelinated, which can result in reduced signal amplitudes and latency delays in the exchange of electrical signals. The simultaneous increase in impedance due to glial scar formation and reduced neuronal viability or function due to lack of oligodendrocyte or myelin support can affect the recording and stimulating potential of the tissue surrounding the electrode. Chronic injuries will leave tissue unrepairable as remyelination or regeneration of axons fails to occur.