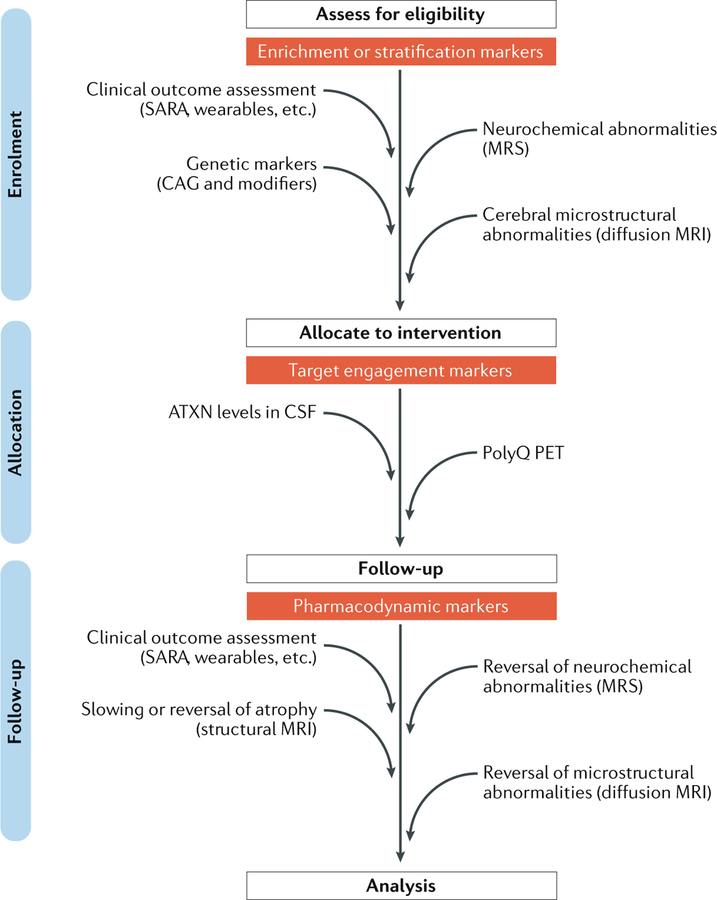
Fig. 6 |. Biomarkers for SCAs.
Diagram shows the stages at which clinical outcome assessment measures and biomarkers are expected to have utility in a hypothetical gene-silencing trial of a polyglutamine (polyQ) spinocerebellar ataxia (SCA) in which a patient cohort with early and premanifest disease is targeted. Clinical outcome assessment, genetic markers (for example, size of CAG triplet repeat and other modifiers of age of onset) and MRI or magnetic resonance spectroscopy (MRS) markers might facilitate the selection of patients with minimal cerebral involvement and enable premanifest enrolment of patients who already show cerebral changes. Measures of the disease protein in cerebrospinal fluid (CSF) are being developed to monitor target engagement in those who have been allocated to a gene-silencing intervention. Use of a PET tracer that specifically binds to polyQ repeats could provide direct evidence of clearance of the mutant protein or aggregates from the brain195. Finally, MRI and MRS markers are expected to aid treatment monitoring as secondary outcome measures that supplement the primary clinical outcome assessment measures. Furthermore, MRI and MRS markers might also serve as safety markers to ensure that the treatment does not exacerbate the cerebral pathology. The biomarkers shown are not intended to be comprehensive and need further development and/or validation, particularly in the premanifest and early disease stages, for the proposed uses in trials. ATXN, ataxin; SARA, Scale for Assessment and Rating of Ataxia.