Fig. 3. Details of the HDH complex structure.
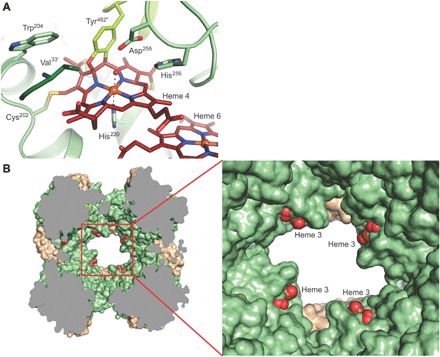
(A) Close-up of the active site. Three monomers, shown in different colors, contribute side chains to the active-site cavity, such as the conserved Asp255/His256 pair from one monomer and the N-terminal Val33′ from another. The active-site heme 4 is bound covalently to Tyr462″ from a third monomer, as well as to the conserved Cys202 in addition to the two cysteines of the heme-binding motif (in the background). (B) Sliced view of the HDH complex. Heme groups 3 of each monomer are solvent-exposed just inside the holes, leading to the central cavity in the complex.
