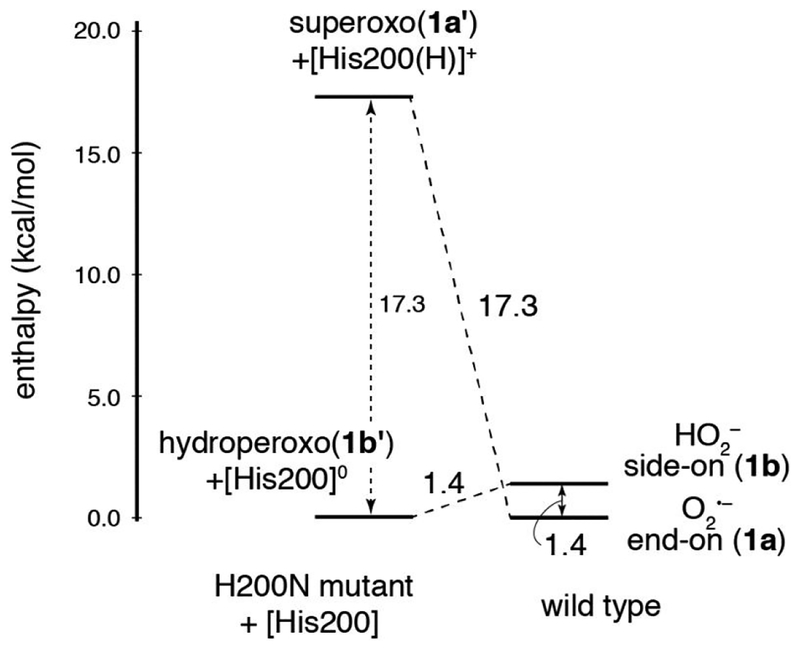
Figure 6.
Energy diagram showing the effect of H200 on the relative enthalpies of superoxy and hydroperoxy intermediates in HPCD. The energetics of wild-type 1a and 1b are shown on the right, and 1a’ + [H200(H)]+ and 1b’ + [H200]0 are shown on the left (which include a non-interacting H200 in a separate calculation as a proton donor/acceptor). Removing H200 destabilizes the FeIII-superoxo intermediate by 17.3 kcal/mol and stabilizes the FeIII-hydroperoxo intermediate by 1.4 kcal/mol. Here, enthalpy is shown rather than Gibbs free energy due to entropic differences introduced for the structures on the left through removal of the H200. Energies have been corrected for BSSE.