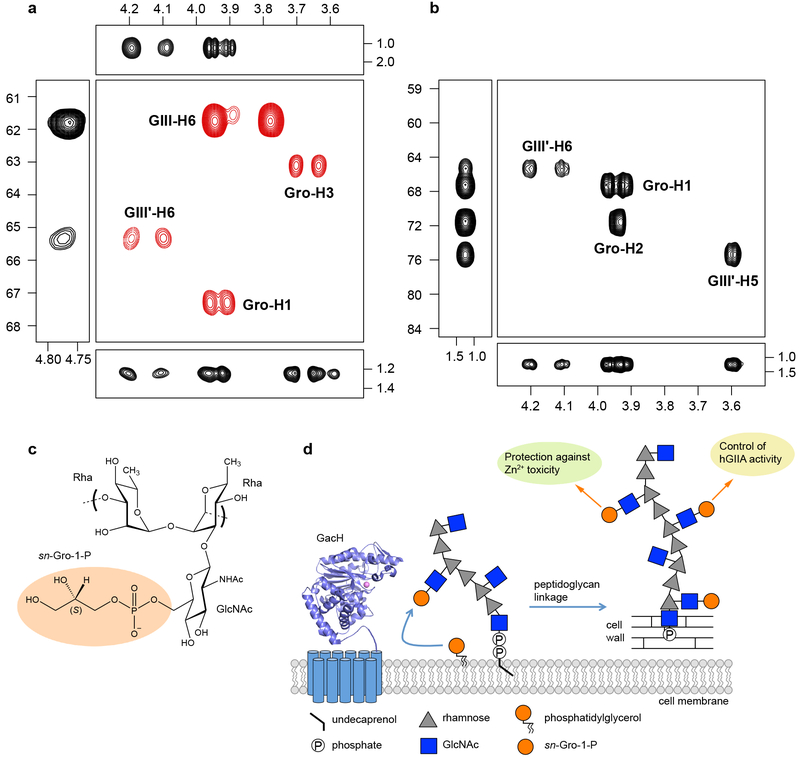
Fig. 5. NMR analysis confirms presence of GroP on C6 GlcNAc hydroxymethyl group of GAC.
a–b, Selected regions of NMR spectra of GAC. a, Multiplicity-edited 1H,13C-HSQC in which methylene groups have opposite phase and are shown in red color (center box), 1H,13C-HSQC-TOCSY with an isotropic mixing time of 120 ms (left box), 1H,13C-HMBC with a mixing time of 90 ms (top box), 1H,31P-hetero-TOCSY with an isotropic mixing time of 80 ms (bottom box). b, 1H,13C-plane (center box), 13C, 31P-plane using a nominal nJCP value of 5 Hz (left box), and 1H, 31P-plane (bottom box) of a through-bond 3D 1H,13C,13P NMR experiment. Cross-peaks are annotated as GIII corresponding to the GlcNAc residue, GIII' being the GroP-substituted GlcNAc residue and Gro as the glycerol residue. NMR chemical shifts of 1H (horizontal axis), 13C (left axis) and 31P (right axis and left box in b) are given in ppm. Experiments depicted in a–b were performed independently three times and yielded the same results. c, Schematic structure of the GAC repeating unit consisting of →3)-α-l-Rhap-(1→2)[β-d-GlcpNAc6P(S)Gro-(1→3)]-α-l-Rhap-(1→. d, The mechanism and the roles of GroP cell wall modification in streptococci.