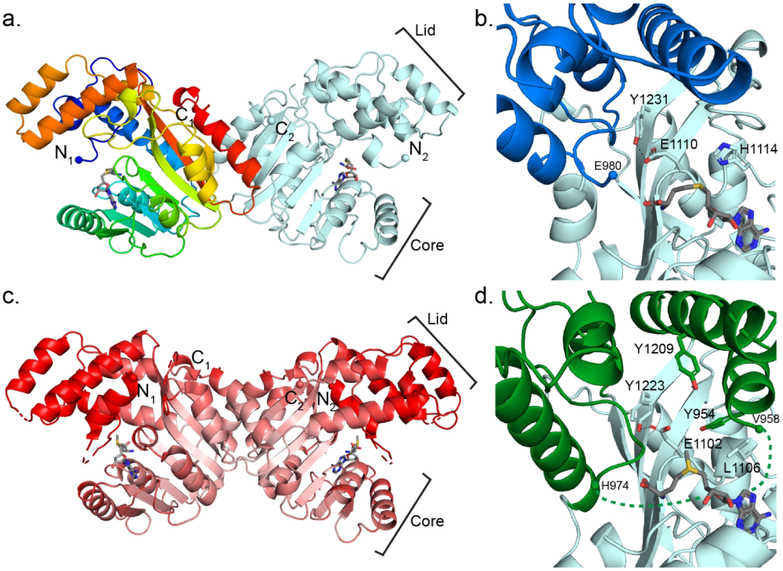
Figure 3.
StiD and StiE O-MT structures. (a) StiD O-MT dimer. One protomer is colored as a rainbow from blue (N-terminus) to red (C-terminus). SAH is shown in sticks with atomic coloring (C, gray; O, red; N, blue; S, yellow). Termini are shown in spheres. (b) StiD O-MT active site colored by structural region (core, light blue; lid, dark blue). Key amino acids and SAH are shown in sticks with atomic coloring. The first ordered residue in the crystal structure (Glu980) is indicated with a sphere. (c) StiE O-MT dimer colored according to Cα B-factor from red (80 Å2) to white (20 Å2). SAM is shown in sticks. In both StiE and StiD, the lid and N-terminal helix have far greater mobility than the SAM binding core. (d) StiE O-MT active site colored by structural region (core, light blue; lid, green). Key amino acids and SAM are shown in sticks with atomic coloring. The last amino acid (Val958) of the N-terminal helix containing conserved aromatic amino acid Tyr954 is 17 Å away from the next ordered residue (His974).