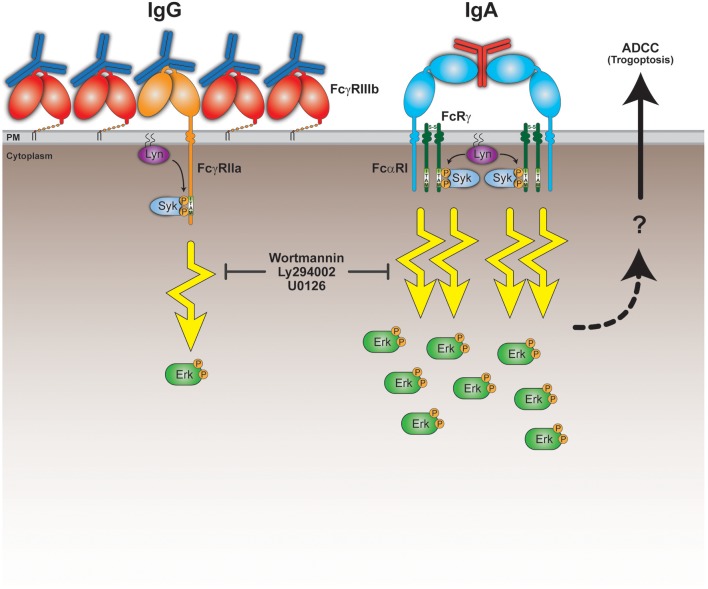
Figure 5.
Model for superior IgA-mediated tumor cell killing by neutrophils. Neutrophils express the low affinity Fc receptors FcγRIIIb, FcγRIIa, and FcαRI. Both FcγRIIa and FcαRI are activating Fc receptors, because upon ligand (antibody Fc domain) binding, clustering and recruitment of kinases (e.g., Lyn), they signal through their ITAM domains and elicit cellular effector functions. The relatively high FcγRIIIb expression on neutrophils could possibly interfere with the activating capacity of FcγRIIa by competing for Fc domain binding and preventing proper formation of signaling platforms and/or organization within the lipid bilayer. This results in a poor ability of FcγRIIa to initiate sufficient signaling in neutrophils. Despite the low FcαRI expression on neutrophils, they can still engage clustered IgA Fc domains to a similar degree as IgG Fc domains bind the FcγRs. IgA can, however, bind FcαRI bivalently resulting in more stable binding and recruitment of in total 4 ITAMs. This scenario would initiate a robust ITAM signaling necessary for activating effector functions, including trogoptosis to eliminate tumor cells. This is further illustrated by the signaling inhibitors wortmannin, Ly294002 and U0126 that prevent tumor cell lysis in vitro. The question mark refers to yet unclear processes involving signaling, intracellular Ca2+, actin-myosin contraction, and immune cell-tumor cell interactions that lead to ADDC by neutrophils (trogoptosis) (23).