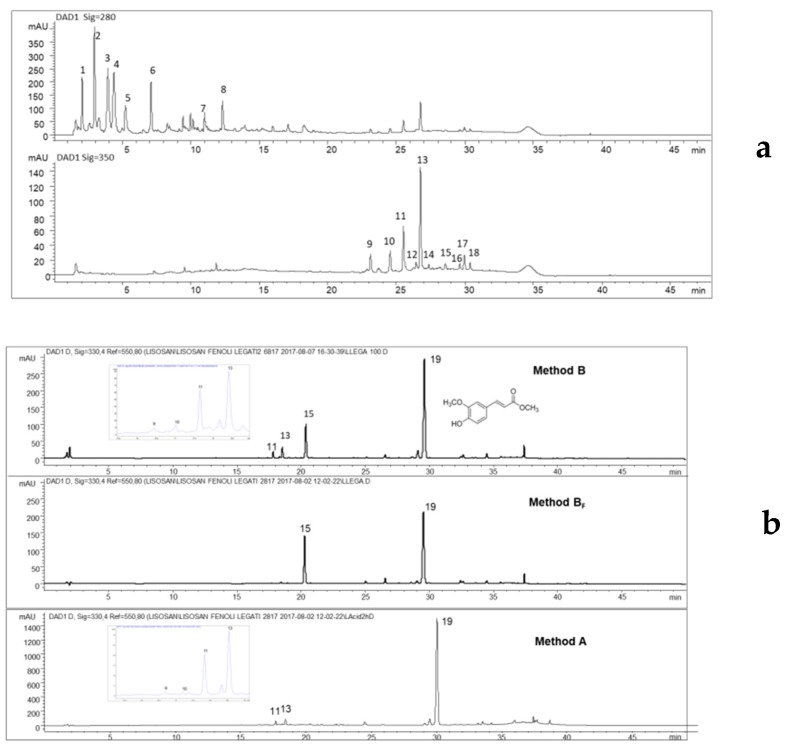
Figure 1.
(a) Chromatographic profiles at 280 and 350 nm of the aqueous extract of Lisosan® G on the Poroshell column, obtained applying the same gradient elution used for the semipreparative HPLC. Compounds 1–8, unknowns; 9 and 10, neocarlinoside or its isobars, isocarlinoside/carlinoside; 11, isoschaftoside; 13, schaftoside; 15, ferulic acid; 16–18 unknowns; and (b) comparison of the HPLC profiles at 330 nm obtained for bound phenols with methods B and A on the whole flour, method BF on the residue from free phenol extraction; 9 and 10, neocarlinoside/isocarlinoside/carlinoside; 11, isoschaftoside; 13, schaftoside; 15, ferulic acid; 19, methyl ferulate and its chemical formula.