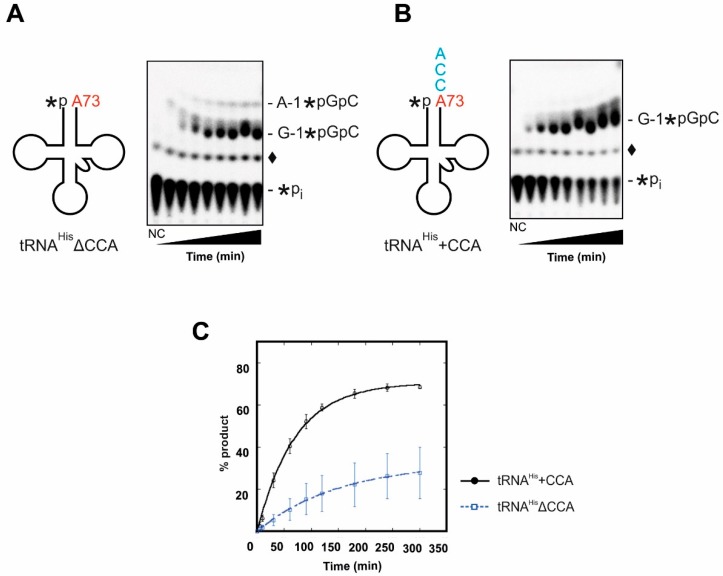
Figure 3.
The CCA triplet affects the fidelity of G-1-addition by Thg1. 5′-labeled tRNAHis lacking the CCA-end (tRNAHisΔCCA); (A) or ending with CCA (tRNAHis+CCA); (B) were incubated with saturating amounts of Thg1 (15 µM) and assayed using a phosphatase protection assay, in which the 5′-32P-label on unreacted tRNA substrate is accessible to phosphatase, and visualized as inorganic phosphate (*Pi). On tRNAHisΔCCA, Thg1 exhibits a reduced fidelity and adds not only the correct G-1 (G-1*pGpC product) but to a certain amount also erroneously A-1 (A-1*pGpC product on panel A). However, when the tRNA substrate carries a 3′-terminal CCA sequence, Thg1 exclusively incorporates G-1, indicating that the CCA triplet contributes to the fidelity of the reaction. An additional non-enzymatic labeled species (♦) is visible in the no enzyme control (NC) and enzyme-containing reactions, as has been observed previously with these types of labeled tRNA assays [13,23]. (C) Single-turnover nucleotide incorporation was measured in triplicate and plotted as a function of time. 5′-labeled tRNAHisΔCCA (blue) and tRNAHis+CCA (black) were incubated with 15 µM Thg1 in the presence of 0.1 mM ATP and 1 mM GTP.