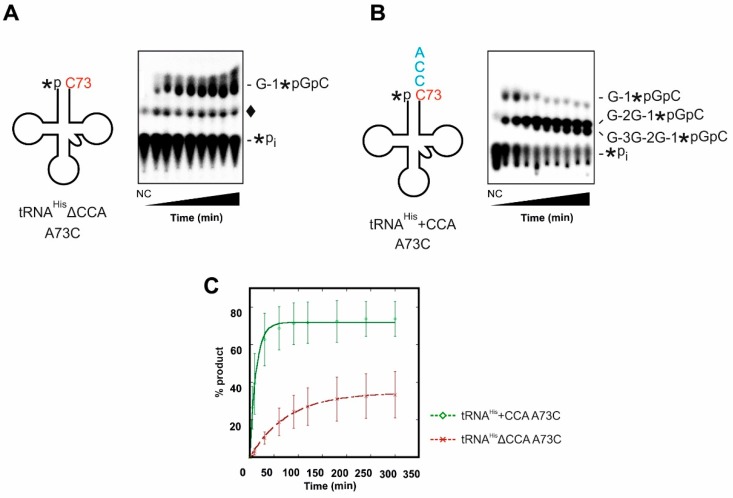
Figure 4.
Thg1 activity on cytosolic tRNAHis carrying a cytosine residue at the discriminator position 73 (red). (A) On tRNAHisΔCCA A73C, the enzyme correctly adds a single G-1 residue (G-1*pGpC product), consistent with the absence of a 3′-end template sequence for further 3′-5′ polymerization. Other species observed in this assay include *Pi, which represents the remaining unreacted substrate tRNA, and ♦ which represents the non-enzymatic product that is visible in both no enzyme control (NC) and enzyme-containing reaction lanes. (B) If tRNAHis A73C additionally carries the 3′-CCA-end (cyan), Thg1 catalyzes multiple GTP incorporations in a 5′-template-dependent manner, resulting in two additional G-C base pairs with the 3′-CCA-end (G-2G-1*pGpC and G-3G-2G-1*pGpC products). In this case, the non-enzymatic side product is not visible because it is obscured by the strong signal from the multiple G-addition products. (C) Single-turnover nucleotide incorporation was measured in triplicate and plotted as a function of time. 5′-labeled tRNAHisΔCCA A73C (red graph) or tRNAHis+CCA A73C (green graph) were incubated with saturating amounts of enzyme (15 µM), 0.1 mM ATP and 1 mM GTP.