Figure 5.
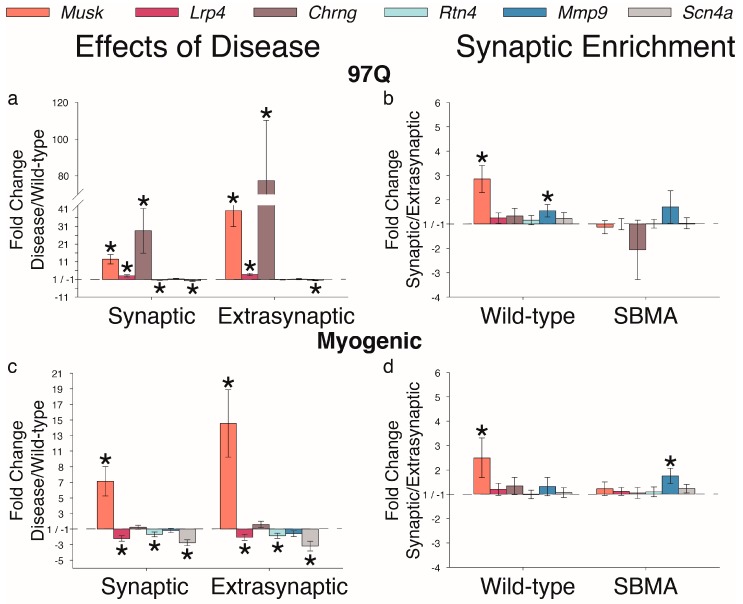
Disease affects genes implicated in synaptic stability and function. (a,c) With few exceptions, synaptic-related genes are affected by disease in one or both disease models. Changes include a marked increase in Musk, which encodes a receptor tyrosine kinase for the AChR-stabilizing agrin, and Chrng, encoding the neonatal subunit of the AChR. Disease also affects Lrp4 in both models although in divergent directions—being increased in the 97Q model while decreased in myogenic model. Other significant and consistent changes in transcript levels include a downregulation of Rtn4, encoding an inhibitory signal for axonal sprouting, and Scn4a, encoding the sodium voltage-gated channel alpha subunit 4, the adult isoform which controls the influx of sodium into muscle cells. (b,d) Notably, the synaptic enrichment of Musk is also lost with disease. Thus, the challenges of disease seem to trigger an adaptive response to maintain and/or rescue neuromuscular synaptic function by reverting back to a permissive environment for axon sprouting (by downregulating Rtn4) and by increasing AChR expression and recruiting and stabilizing these newly made AChRs at the synapse (by upregulating Musk and Chrng). These data underscore the general theme that many genes important for synaptic stability are dysregulated in muscles of SBMA mice, possibly explaining the characteristic deficit in synaptic strength. Fold changes ± SEM are shown relative to wild-type (a,c) or extrasynaptic (b,d) samples; Statistical analysis was based on pair-wise fixed reallocation randomization test: * p < 0.05.