Figure 1.
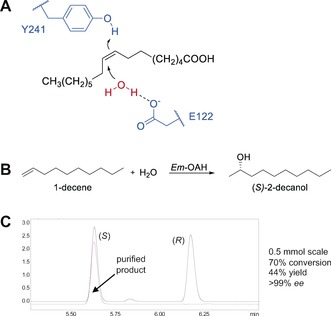
Asymmetric hydration of aliphatic alkenes using Em‐OAH. A) Postulated reaction mechanism of the Em‐OAH‐catalyzed hydration of oleic acid. The protonation of the carbon–carbon double bond at the C9 atom of oleic acid is initiated by Y241, followed by a nucleophilic attack of a water molecule that is activated by E122.32 B) Enantioselective hydration of 1‐decene by Em‐OAH wildtype enzyme using heptanoic acid as a decoy molecule. C) GC analysis on a chiral support, and results for the preparative‐scale reaction of 1‐decene (red line in GC trace). Upscaling of 1‐decene hydration by Em‐OAH wildtype was performed with 70 mg starting material (2 mm 1‐decene) using a whole‐cell catalyst (100 mg mL−1) and heptanoic acid as a decoy molecule.
