Table 1.
Photophysical properties of 4,5‐dihydropyridazine products.
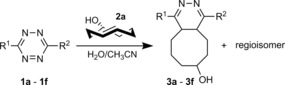
| |||||
|---|---|---|---|---|---|
| λ abs/λ em [a] | Stokes | Φ fl [b] | Intensity | ||
| R1 | R2 | [nm] | shift | [%] | increase |
| [nm] | |||||
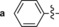
|
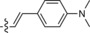
|
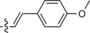
545/628 | 83 | 0.55 | 13‐fold |
|
|
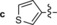
295/– | – | – | – |
|
|
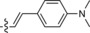
546/626 | 80 | 0.52 | 18‐fold |
|
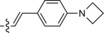
|
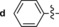
546/626 | 80 | 0.45 | 10‐fold |
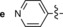
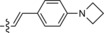
|
|
566/643 | 77 | 0.21 | threefold |
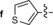
|
|
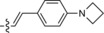
549/626 | 77 | 0.47 | 12‐fold |
[a] Absorption and emission maxima were measured in CH3CN/H2O 1:1. [b] Relative fluorescence quantum yields were determined by using Nile Red in methanol as standard.
