Abstract
Maltodextrins (MD) are frequently used as processing aids in tomato drying. The aim of this study was to investigate the effect of the addition of MD on the stability of lycopene and chlorogenic acid, which are the main lipophilic and hydrophilic antioxidants in processed tomato, respectively. Tomato powder added with 10% MD (dextrose equivalents, DE 12) and a control tomato powder were stored in the water activity (aw) range 0.17–0.56, for 180 d at 30 °C. At the aw level of 0.17, which was below the monolayer moisture content (Mo), chlorogenic acid was stable, while lycopene content decreased faster in tomato added with MD than in control tomato, probably due to a decrease in matrix hydrophilicity and greater oxygen diffusion in the oil phase. Maximum stability occurred in both tomato powders at aw of 0.3, that was in close proximity to Mo (first-order rate constant for lycopene, k = 7.0 × 10−3 d−1 in tomato added with MD). At high aw levels, MD increased the rate of lycopene degradation with respect to the control, possibly by hampering its regeneration by chlorogenic acid, which conversely was found to be more stable than in the control tomato.
Keywords: maltodextrins, lycopene, chlorogenic acid, water activity, kinetics
1. Introduction
It is well established that the consumption of tomato is associated with various health benefits ranging from cardiovascular health to protection against cancers [1,2,3]. These health properties have been proven in a number of epidemiological studies [4,5] and in vitro model systems. The mechanisms underlying tomato health effects depend mainly on lycopene, which is its peculiar lipophilic antioxidant. Lycopene is a highly unsaturated acyclic carotenoid, which exhibits a high physical quenching rate of singlet oxygen [6]. Protective effects of lycopene against oxidative stress have been reported in a number of studies [7,8]. Moreover, tomatoes are a source of both ascorbic acid and phenolic compounds, mainly chlorogenic acid, which are common hydrophilic antioxidants among vegetables, which are also regarded as important bioactive compounds [9,10,11].
Since humans cannot synthesize these compounds de novo, and considering the beneficial health effects they impart, their content in raw and processed tomato has become an important area of research. Lycopene is stable upon thermal processing of tomato. Moreover, the thermal processes applied to tomato can result in the breakdown of cell walls, thus improving lycopene bioaccessibility. Indeed, numerous studies have shown that lycopene bioavailability is higher for processed tomato than for fresh tomato [12]. Conversely, ascorbic acid is very sensitive to thermal degradation, while the content of phenolic compounds in tomato increases after thermal treatments, due to solubilization from the plant cellular wall as well as inactivation of polyphenol oxidase [10].
One of the main technologies applied to tomato is drying. Lycopene and chlorogenic acid are not affected by drying but their stability is low during storage of the dehydrated product [13]. Maltodextrins (MDs) are frequently used as processing aids in tomato drying. MDs are among the most important carrier agents in the spray-drying process, mainly because they form low viscosity solutions in high concentrations that can be efficiently spray-dried [14]. MDs with DE 6–21 were applied in the spray-drying of tomatoes, resulting in increased efficiency of the process due to the MD ability to encapsulate low molecular weight sugars and reduce wall deposition problems [15]. Moreover, MDs are used for tomato puree foam drying [16].
MDs are also applied as osmotic agents in osmotic dehydration, which results in the development of intermediate moisture products having a lower water activity (aw) imparted by solute gain and water loss. Osmotic dehydration is performed as a pre-drying treatment at low temperatures, because it is a less energy-intensive process than other drying processes [17]. Following this latter approach, a solution of 27.5% MD with DE 10 and 10.0% NaCl, combined with the application of a vacuum pulse (100 mbar, 20 min) was used at 40 °C for the osmotic dehydration of tomatoes. The use of MD decreased NaCl incorporation and increased the effective diffusivity of water, thus accelerating the osmotic dehydration process [18]. Moreover, MDs have been applied as encapsulating agents for phenolic compounds, providing protection against oxidative degradation during processing and storage of foods [19].
Despite the common use of MDs in various drying technologies, little information is available on the effect of MDs on the stability of the main antioxidants of tomato. Previous studies revealed low stability of lycopene in starch or MDs added matrices. In fact, soluble starch was used as a carrier for the encapsulation of lycopene from Rosa rubiginosa by spray-drying, resulting in a half-life of 5 d at 21 °C [20]. The peel fraction of tomato mixed with MD (DE not specified) before spray-drying showed a half-life of 12 d [21]. In this latter study a control matrix without MD was not run in parallel, thus the specific effect of MD on lycopene stability cannot be derived. It is worth noting that the kinetics of lycopene degradation in dried matrices is greatly affected by the water activity (aw) level [13,22]. Moreover, the presence of MDs affects the water sorption properties and the stability of dry foods [23]. Hence, the identification of the optimal aw level to achieve upon drying is crucial to extend storage stability.
As food supply chains are constantly evolving toward healthier food production and better resource management, food technologies should combine the production of foods with promising bioactive compounds with sustainable processes [24]. From a sustainability perspective, drying, which is notably an energy-consuming operation, should be followed by storage at ambient temperature which prevents the need for energy-consuming cold storage facilities.
Therefore, the aim of this study was to set kinetic models for lycopene degradation in dried tomato matrices added with MD (DE 12) and to find the aw conditions for maximum storage stability. The degradation reactions occurring in both the polar and the oil phases of the tomato matrix were investigated and a model for oxidative phenomena that can be useful for the stability optimization of lycopene-rich food matrix was proposed.
2. Results and Discussion
2.1. Matrix Composition and Hygroscopicity
The composition of tomato pulp powder is shown in Table 1 and moisture isotherms of tomato products, fitted by the Guggenheim–Anderson–de Boer (GAB) equation, are shown in Figure 1.
Table 1.
Major components of tomato pulp powder (g/100 fresh weight.)
| Quality Index | Percent Content |
|---|---|
| moisture | 8.6 ± 0.2 |
| protein | 8.8 ± 0.9 |
| fat | 1.9 ± 0.2 |
| insoluble dietary fiber | 15.7 ± 1.2 |
| soluble dietary fiber | 2.7 ± 0.4 |
| glucose + fructose | 34.0 ± 1.6 |
| ash | 6.4 ± 0.5 |
| titratable acidity | 0.20 ± 0.01 |
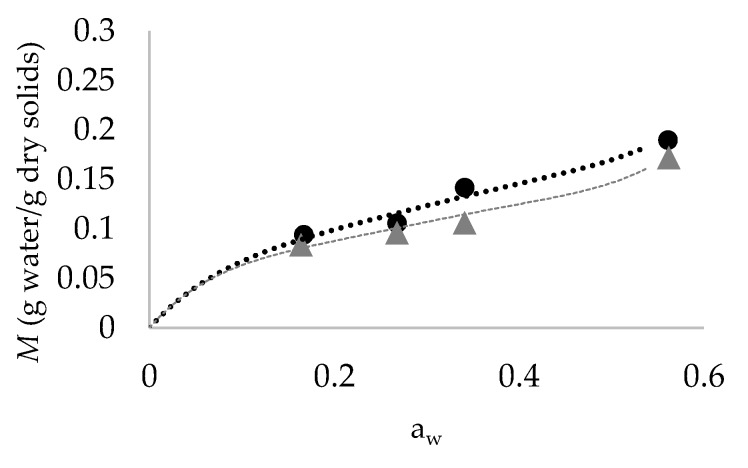
Figure 1.
Equilibrium moisture content (M) as a function of water activity (aw) for tomato pulp powder (black line and symbols) and tomato pulp powder added with maltodextrins (MD) (grey line and symbols). Full symbols represent experimental data for samples equilibrated over saturated salt solution in a desiccator at 30 °C, lines represent adsorption isotherm obtained by fitting experimental data with the Guggenheim–Anderson–de Boer (GAB) model.
The tomato pulp powder isotherm was best described as a Brunauer type II isotherm, which is associated with relatively strong interactions between absorbent and absorbate, and results from multi-layer absorption of water, capillary filling, and capillary condensation [25]. Sugars and organic acids present in tomato pulp powder likely contribute to its hygroscopicity, indeed tomato skin powder, which possesses more fiber and waxy cuticle materials is less hygroscopic than the tomato pulp powder [25].
As expected, the addition of 10% MD slightly decreased tomato hygroscopicity, since at a given aw the amount of water adsorbed was always lower for tomato pulp powder added with MD than for tomato pulp powder (Figure 1). Mo, C and K values were found to be 0.120 g water/g dry solids, 13 and 0.76 for tomato pulp powder and 0.099, 18 and 0.86 for tomato pulp powder added with MD, respectively. Mo corresponded to an aw level of 0.28 for tomato pulp powder and 0.26 for tomato pulp powder added with MD. A slightly higher Mo value for tomato pulp powder was observed by Xu et al. [25], i.e., 0.16 g waters/g dry solids.
2.2. Kinetics of Lycopene Degradation
During storage, lycopene content decreased in both tomato pulp powder and tomato pulp powder added with MD by following first-order kinetics (Figure 2, Table 2).
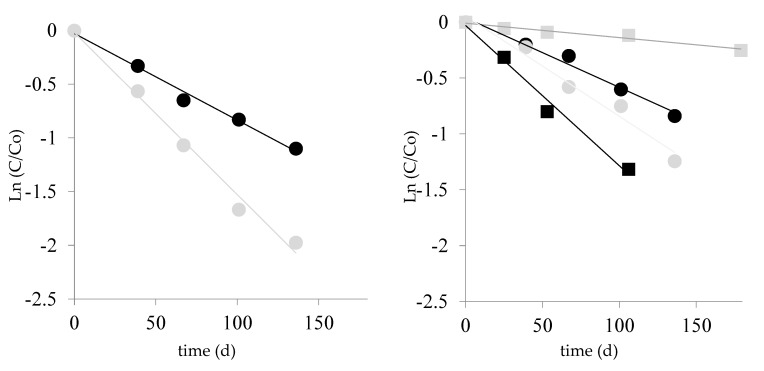
Figure 2.
Time course of lycopene (circle) and chlorogenic acid (square) degradation in tomato pulp powder (black line and symbols) and tomato pulp powder added with MD (grey line and symbols) during storage at aw 0.17 (on the left) and 0.56 (on the right). Symbols represent experimental data; lines represent fitting experimental data with first-order kinetics.
Table 2.
Initial concentration (mg/kg dry weight), storage temperature (°C), time (d), aw, first-order rate constant (d−1) and half-life (d) of lycopene in various matrices.
| Matrix | Co | T | t | aw | k × 103 | t1/2 | Ref. |
|---|---|---|---|---|---|---|---|
| Tomato peel, freeze-dried | 7390 ± 70 | 30 | 139 | 0.17 | 19 ± 1.2 | 41 | Lavelli et al. [13] |
| 0.22 | 10 ± 1.5 | 63 | |||||
| 0.32 | 9.0 ± 1.1 | 81 | |||||
| 0.56 | 5.0 ± 0.8 | 115 | |||||
| Tomato concentrate + MD, spray-dried | 494 ± 10 | 25 | 28 | nd | 57.6 | 12 | Souza et al. [21] |
| Tomato pomace extract + poly-γ-glutamic, freeze-dried | 134.2 ± 2.3 | 35 | 30 | nd | 24.7 | 28 | Chiu et al. [27] |
| Tomato oleoresin + zein, spray-dried | nd | 25 | 18 | nd | 63.6 | 10.9 | Xue et al. [28] |
| Lycopene in oil +modified starch, spray-dried | 5000 | 25 | 78 | nd | nd | >78 | Rocha et al. [29] |
| Tomato pulp powder, freeze-dried | 2465 ± 20 | 30 | 139 | 0.17 | 8.1 cd ± 0.5 | 86 | 1 This study |
| 0.22 | 7.7 cd ± 0.5 | 90 | |||||
| 0.32 | 5.8 a ± 0.2 | 119 | |||||
| 0.56 | 6.2 ab ± 0.3 | 111 | |||||
| Tomato pulp powder + MD, freeze-dried | 2184 ± 20 | 30 | 139 | 0.17 | 15.0 e ± 0.8 | 46 | 1 This study |
| 0.22 | 7.0 bc ± 0.9 | 99 | |||||
| 0.32 | 7.6 c ± 0.6 | 91 | |||||
| 0.56 | 8.8 d ± 1.2 | 79 |
1 Different letters (a–e) indicate significant differences among first-order rate constants (LSD, p < 0.05).
Carotenoid degradation has been reported to follow radical-based reactions. These reactions could be initiated by transition metal ions. In fact, electron transfer occurs between transition metal ions like ferric iron and a carotenoid compound, forming the ferrous ion and the carotenoid radical cation that can start radical reactions, leading to further carotenoid loss [26]. Since lycopene is lipid soluble, its degradation could also be triggered by the autoxidation of unsaturated fatty acids, which in turn could be initiated by transition metal ions. In the presence of lipid alkyl, alkoxy and peroxy radicals, three types of reactions can involve the carotenoid compounds, namely: electron transfer reactions to form radical cations, reaction with radicals through hydrogen abstraction, or adduct formation reactions [26].
In freeze-dried tomato pulp powder, initial lycopene content was 2465 mg/kg on dry weight basis (d.w.) (Table 2). The degradation rate for lycopene in the pulp powder decreased with increasing moisture content, with half-lives at 30 °C increasing from 86 to 119 d when the aw level increased from 0.17 to 0.32. The addition of MD to tomato pulp powder greatly decreased lycopene stability, especially at the lowest aw of 0.17, where the half-life was 46 d, half than that observed in the absence of MD. Similarly, lycopene stability in tomato peel was very low at the aw of 0.17, with a half-life of 41 d [13].
In previous studies, encapsulation of the lipid matrix containing lycopene was proposed to improve lycopene stability. Use of soluble starch and MD as a carrier before spray-drying of lycopene oleoresin and tomato peel, resulted in a half-life of 5 d and 12 d at 21–25 °C [20,21], respectively. The fast degradation rate of lycopene was also found by Chiu et al. [27] for the freeze-dried extract of tomato pomace obtained by supercritical carbon dioxide encapsulated in poly-γ-glutamic acid (aw not specified), resulting in a half-life of 25 d at 30 °C. Tomato oleoresin was also encapsulated in zein by spray-drying. By this approach, the stability was even lower, resulting in a half-life of 10.9 d at 25 °C [28].
Hence, none of the hydrophilic polysaccharides or proteins proposed as a lycopene encapsulating agent was effective in increasing lycopene stability during storage. It may also be concluded that lycopene was not effectively surrounded by the hydrophilic polymers. In fact, only chemical modification of starch with the incorporation of a lipophilic compound was found as a good encapsulation agent for lycopene, with retention higher than 50% after storage for 78 d at 25 °C [29].
2.3. Kinetics of Chlorogenic Acid Degradation
At aw levels <0.32 no changes were observed in the content of chlorogenic acid of tomato products. Conversely, at aw 0.56 chlorogenic acid content of tomato pulp powder decreased greatly, by following first-order kinetics with a half-life of 58 d (Figure 2, Table 3). It was previously observed that the rate of degradation of chlorogenic acid increases with an increase in aw at values ≥0.55. This finding was related to an increase in molecular mobility, as assessed by 1H NMR [30].
Table 3.
Initial concentration (mg/kg dry weight), storage temperature (°C), time (d), aw, first-order rate constant (d−1) and half-life (d) of chlorogenic acid in various matrices.
| Matrix | Co | T | t | aw | k × 103 | t1/2 | Ref. |
|---|---|---|---|---|---|---|---|
| Apple pulp powder | 1050 ± 20 | 30 | 30 | 0.56 | 2.0 | 347 | Lavelli et al. [30] |
| Low-methoxyl-pectin film | 10.0 ± 0.6 | 25 | 215 | 0.58 | 3.7 ± 0.6 | 186 | Basanta et al. [31] |
| Tomato pulp powder, freeze-dried | 104 ± 18 | 30 | 106 | 0.17 | n.s. | This study | |
| 0.22 | n.s. | ||||||
| 0.32 | n.s. | ||||||
| 0.56 | 12 ± 1 | 58 | |||||
| Tomato pulp powder +MD, freeze-dried | 171 ± 3 | 30 | 106 | 0.17 | n.s. | This study | |
| 0.22 | n.s. | ||||||
| 0.32 | n.s. | ||||||
| 0.56 | 1.3 ± 0.2 | 533 |
The half-life of chlorogenic acid in apple pulp powder stored at aw 0.54 at 30 °C was found to be 347 d [30], while in low-methoxyl-pectin (LMP) film stored at aw 0.58 at 20 °C it was found to be 186 d [31]. Apple pulp powder has a low content of fat (0.46% d.w.), [30]. The lower stability of chlorogenic acid found in tomato pulp powder with respect to apple pulp powder could be attributed to its higher level in fats (2.0% d.w.), which can trigger radical-based reactions.
The presence of MD in tomato pulp powder was associated with significantly enhanced stability of chlorogenic acid, which showed a half-life of 533 (Table 3). MDs have been reported to encapsulate chlorogenic acid [32]. This could be the reason for their protecting effect.
2.4. Overview of Degradation Phenomena in the Water and Oil Compartments
Based on the results obtained, a model of oxidation phenomena occurring in both the polar and the oil phases of tomato pulp powder was developed.
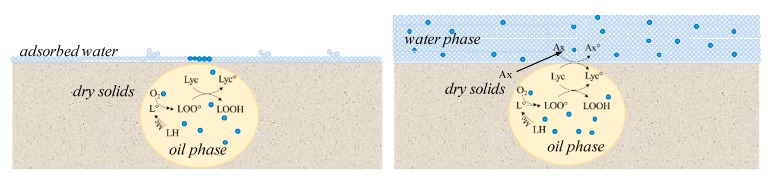
As a general rule, at aw of 0.17 no changes were observed in the polar antioxidant content—i.e., chlorogenic acid—while the lowest lycopene stability was observed. At this aw level, moisture content was below Mo. Since tomato pulp powder is a heterogeneous matrix, below Mo, water molecules are bound to the most hydrophilic sites of the dried solids. Under these conditions, water molecules are likely in a monolayer arrangement, but clustering of water molecules is also possible when the affinity of water towards dry solids is lower relative to cohesion between water molecules (Figure 3). Whatever the arrangement of water molecules, below Mo, part of the dry solid surface is directly exposed to air. As for moisture, oxygen sorption occurs on the dry solids [33]. It is likely that preferential sites for oxygen sorption are the most hydrophobic sites of the dry solids, where moisture is excluded. Moreover, the dry solids can contain a different amount of void volumes, depending on the drying process. According to Prado et al. [34], at low water content, the nonpolar carotenoid degradation rate is enhanced by the porosity of the matrix. In this hypothesis, Harnkarnsujarit et al. [35] studied the microstructure of MD and sugar matrices in freeze-dried systems by scanning electron microscopy and demonstrated that freezing of MD without sugars formed larger pore sizes in the freeze-dried solids than in the presence of sugar (glucose, fructose, and sucrose). Additionally, MDs were proven to be a very good encapsulating agent for low molecular weight sugars such as fructose and organic acids [36]. It may be hypothesized that addition of MD to tomato pulp powder caused the formation of larger pore sizes during freeze-drying and decrease the hydrophilicity of the matrix due to sugar encapsulation, thus favoring oxygen binding. This could explain the decrease in lycopene stability upon MD addition to tomato. On the contrary, the presence of low-molecular-weight sugars in tomato pulp powder without MD was crucial to increase lycopene stability.
Figure 3.
Model for oxidative reactions in dry tomato powder at aw below the monolayer moisture (Mo) (left side) and at aw well above Mo (right side).  = oxygen,
= oxygen,  = water; Lyc = lycopene, Ax = hydrophilic antioxidant, LH = unsaturated fatty acid, L° and LOO° = radicals, LOOH = peroxide. At aw below Mo, MD could enhance oxygen binding due to decreased hydrophilicity of the matrix and larger pore sizes, thus increasing lycopene degradation rate. At aw well above Mo, MD could encapsulate the hydrophilic antioxidants thus hampering lycopene regeneration.
= water; Lyc = lycopene, Ax = hydrophilic antioxidant, LH = unsaturated fatty acid, L° and LOO° = radicals, LOOH = peroxide. At aw below Mo, MD could enhance oxygen binding due to decreased hydrophilicity of the matrix and larger pore sizes, thus increasing lycopene degradation rate. At aw well above Mo, MD could encapsulate the hydrophilic antioxidants thus hampering lycopene regeneration.
At the aw levels of 0.22 and 0.32 the rate for lycopene degradation decreased. These aw levels are in close proximity to the monomolecular moisture content. The decrease in degradation rate could be due to the small amount of water that combines metal ions and precipitate them as insoluble hydroxides [13]. Additionally, water covers the solid surface. This means that oxygen molecules have to diffuse through the layers of water clusters first, before they can come into contact with the sorption sites on solids’ surface. Water is a poor solvent for oxygen [33]. Indeed, oxygen solubility is of 8–10 mg/L [37]. Consequently, the amount of oxygen absorbed to the matrix decreases with respect to that absorbed at aw 0.17.
At aw 0.56, the stability decreased in the hydrophilic compartment of the tomato pulp powder as shown by the increased degradation rate of chlorogenic acid. Conversely, at aw levels below 0.32, the presence of the hydrophilic antioxidant chlorogenic acid resulted in having no influence on lycopene degradation. In fact, while lycopene degradation was maximum at aw of 0.17, no change occurred in chlorogenic acid content at aw < 0.32. The decrease of chlorogenic acid stability at a 0.55 could be due to the increase in molecular mobility in the water phase, which favors the diffusion of both the oxygen molecules and the oxygen-sensitive targets. Oxygen solubility in oil was reported to be 47 mg/L at 30 °C [37], which is higher than in water. Indeed, vegetable oils solubilize around 4 to 5 times more oxygen than water does, depending on the temperature [38]. On the other hand, the rate for chlorogenic acid degradation in tomato pulp powder at aw 0.55 was much higher than those reported for other matrices with lower or no fat content [30,31]. Moreover, while chlorogenic acid degradation rate increased in tomato pulp powder, the rate of lycopene degradation in the lipid compartment did not increase. It may be hypothesized that an active interaction of antioxidants occurred within and across the hydrophilic and lipophilic compartments of the tomato matrix, as already observed in various model systems [39]. Through this interaction, lipid peroxy and alkoxy radicals and/or lycopene radicals are regenerated by hydrophilic antioxidants such as chlorogenic acid (Figure 3). Indeed, chlorogenic acid was found to be a very efficient scavenger of the radicals formed during lipid peroxidation [40]. However, in the presence of MD the hydrophilic antioxidants were most likely encapsulated in this polymer and could not exert free-radical chain-breaking antioxidant activity, consistent with higher stability of chlorogenic acid but lower stability of lycopene.
3. Materials and Methods
3.1. Preparation of Tomato Pulp Powder
Tomato fruits (about 30 kg) were washed with water and heated for 60 min at 100 °C and homogenized with a food processor for 2 min at maximum speed and then refined using a screw extractor (model 9008 Reber, Luzzara, Italy). The pulp powder was recovered and the pomace (peels and seeds) discarded. The pulp powder was separated into two aliquots. One of these was added with 10% (on dry weight basis, d.w.) of MD D12 and then homogenized with Ultra-Turrax (T25 Janke & Kunkel IKA Labortechnik) for 2 min. The samples were immediately placed on aluminium trays and frozen at –45 for 16 h. Then the samples were freeze-dried (Lyoflex Edwards, Crawley, UK) by applying three subsequent time-temperature steps, namely: −45 °C for 8 h, −20 °C for 24 h, 0 °C for 24 h, and 10 °C for 10 h. The chamber pressure was maintained at 30 Pa throughout the drying process. Then, the powders were ground and sieved (800 μm). The other aliquot did not have MD added. Samples were immediately freeze-dried (Lyoflex Edwards, Crawley, UK)
Freeze-drying was performed at −45 °C for 8 h, −20 °C for 24 h, 0 °C for 24 h, and 10 °C for 10 h. The chamber pressure was maintained at 30 Pa throughout the drying process. Then, the powders were ground and sieved (800 μm).
3.2. Storage Study
Tomato powders were placed into Petri dishes (6 cm diameter, 5.5 g of product in each dish) positioned inside airtight plastic chambers on wire-mesh racks situated above saturated salt solutions. The chambers were stored for 6 months at 30 °C. To create different environments, the following saturated salt solutions were used: LiCl (aw = 0.113 ± 0.002), CH3COOK (aw = 0.216 ± 0.005), MgCl2 (aw = 0.324 ± 0.002) and NaBr (aw = 0.560 ± 0.004). Duplicate chambers were incubated for each aw level. The moisture content and aw after freeze-drying were 8.6 g/100g and 0.17, respectively, with no significant differences between the tomato powders. The samples stored at aw 0.113 maintained their initial aw (0.17) during storage, while all the other samples reached the aw level of the saturated solution in 5 days.
3.3. Moisture Content and aw
The moisture content of the tomato powders equilibrated at the various relative humidity conditions was determined using a vacuum oven at 70 °C and 50 Torr for 18 h. The aw of tomato powders and saturated salt solutions was checked using a dew point hygrometer (Aqualab, Decagon Devices, Pullman, WA, USA). Duplicate determinations were made for each sample.
Moisture isotherms were developed for the tomato pulp powder and tomato pulp powder added with MD by plotting the equilibrium moisture content (M) versus the storage aw. The Guggenheim–Anderson–de Boer (GAB) Equation (1) was used to fit the experimental data:
| (1) |
where M is the equilibrium moisture content on a dry basis (g of water/g of dry solids); Mo is the monolayer moisture content on a dry basis; C and K are constants [13].
3.4. Titratable Acidity
Freeze-dried tomato powders were diluted with water (0.5 g of powder in 20 mL, in duplicate). Titratable acidity was determined by titration with 0.1 M NaOH to pH 8.1. Results were expressed as grams of citric acid per 100 g of dry product.
3.5. Soluble and Insoluble Fiber, Protein, Fat, and Ash
Fiber, protein, fat, glucose, fructose and ash values measured according to AOAC Official Methods of Analysis [41]. Duplicate determinations were made, and results are expressed as milligrams per kilogram of fresh product (f.w.)
3.6. HPLC Equipment
The HPLC equipment consisted of a model 600 HPLC pump coupled with a Waters model 2996 photodiode array detector, operated by Empower software (Waters, Vimodrone, Italy).
3.7. Lycopene
Tomato powders were analyzed monthly up to 139 d. A two-step extraction was applied using tetrahydrofuran (THF) stabilized by the addition of 0.1% butylated hydroxytoluene (2,6-di-tert-butyl-p-cresol, BHT). Aliquots of tomato powders (0.125 g d.w.) were added to 10 mL of stabilized THF. The mixture was vortexed for 1 min and centrifuged (12.000× g at 5 °C for 10 min). The supernatant was recovered into a 25 mL flask. Ten milliliters of stabilized THF was added to the residual solids. The mixture was vortexed for 1 min, stirred for 30 min with a magnetic stirrer, and then centrifuged (12.000× g at 5 °C for 10 min).
The extracts were pooled and brought up to 25 mL with stabilized THF. Extractions were carried out in triplicate. Lycopene content was analyzed by HPLC as described previously [13]. In brief, a Vydac 201TP54 C18 column (250 × 4.6 mm i.d., 5 μm particle size), equipped with a C18 precolumn, was used (Labservice Analytica, Anzola dell’Emilia, Italy). Chromatographic separation was performed with methanol/stabilized THF (95:5) as an eluent under isocratic conditions, 1.0 mL/min flow rate, at room temperature. Peaks were detected at 454 nm. Lycopene was quantified from a calibration curve using a pure standard and expressed as milligrams per kilogram of dry product.
3.8. Chlorogenic Acid
Aliquots of tomato powders (0.25 g dw) were analyzed monthly up to 180 d, except for samples incubated at aw 0.56, which were analyzed up to 106 d. Extraction was performed with 10 mL of methanol. The mixture was vortexed for 1 min, mixed continuously for 30 min with a magnetic stirrer, and then centrifuged at 12.000× g and 5 °C for 10 min. Extractions were carried out in triplicate on initial samples and in duplicate for samples stored at different aw levels. The phenolic contents of methanolic extracts were analyzed by HPLC as described previously [13]. A 250 × 4.6 mm i.d., 5 μm particle size, Symmetry reverse phase C-18 column (Waters) equipped with a Symmetry C-18 precolumn was used. Formic acid (5%) was added to both methanol and water before the following mobile phases were prepared: (A) water/methanol (95:5, v/v); (B) water/methanol (88:12, v/v); (C) water/methanol (20:80, v/v); (D) and methanol. The following gradient elution was used: 0−5 min, 100% A; 5−10 min linear gradient to reach 100% B; 10−13 min, 100% B; 13−35 min linear gradient to reach 75% B and 25% C; 35−50 min linear gradient to reach 50% B and 50% C; 50−52 min linear gradient to reach 100% C; 52−57 min, 100% C; 57−60 min, 100% D. The flow rate was 1 mL/min. Chlorogenic acid was quantified at 330 from calibration curves using pure standard and expressed as milligrams per kilogram of dry product.
3.9. Statistical Analysis
Data were processed using Statgraphics 5.1 (STCC Inc., Rockville, MD, USA). ANOVA, followed by Fisher’s least significant difference test (LSD p ≤ 0.05), was used.
4. Conclusions
In conclusion, the addition of MD to tomato pulp powder greatly enhanced the effect of aw on lycopene stability. At a low aw level below Mo—i.e., when absorption sites were available on the solid surface—addition of MD decreased lycopene stability probably due to a decrease in matrix hydrophilicity and increase in oxygen binding. At high aw levels—i.e., when water covered all the solid surface—MD decreased lycopene stability probably due to encapsulation of the hydrophilic antioxidants, such as chlorogenic acid, thus limiting their ability to regenerate lycopene. Maximum stability occurred at an aw of 0.3, that is in close proximity to Mo. Hence, control of aw of dry tomato pulp powder with MD added can be a strategy to extend lycopene stability at ambient temperature.
Acknowledgments
The authors would like to acknowledge networking support by the COST Action 15136 European network to advance carotenoid research and applications in agro-food and health (EUROCAROTEN).
Author Contributions
Conceptualization and design of study, V.L., methodology and investigation, P.S.C.S.H.; writing—review, discussion, V.L. and P.S.C.S.H.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Mordente A., Guantario B., Meucci E., Silvestrini A., Lombardi E., Martorana G.E., Giardina B., Bohm V. Lycopene and cardiovascular diseases: An update. Curr. Med. Chem. 2011;18:1146–1163. doi: 10.2174/092986711795029717. [DOI] [PubMed] [Google Scholar]
- 2.Scarmo S., Cartmel B., Lin H., Leffell D.J., Welch E., Bhosale P., Bernstein P.S., Mayne S.T. Significant correlations of dermal total carotenoids and dermal lycopene with their respective plasma levels in healthy adults. Arch. Biochem. Biophys. 2010;504:34–39. doi: 10.1016/j.abb.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shanbhag V.K. Lycopene in cancer therapy. J. Pharm. Bioallied Sci. 2016;8:170–171. doi: 10.4103/0975-7406.171740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Denniss S.G., Haffner T.D., Kroetsch J.T., Davidson S.R., Rush J.W., Hughson R.L. Effect of short-term lycopene supplementation and postprandial dyslipidemia on plasma antioxidants and biomarkers of endothelial health in young, healthy individuals. Vasc. Health Risk Manag. 2008;4:213–222. doi: 10.2147/vhrm.2008.04.01.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kristal A.R., Till C., Platz E.A., Song X., King I.B., Neuhouser M.L., Ambrosone C.B., Thompson I.M. Serum lycopene concentration and prostate cancer risk: Results from the Prostate Cancer Prevention Trial. Cancer Epidemiol. Biomark. Prev. 2011;20:638–646. doi: 10.1158/1055-9965.EPI-10-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Mascio P., Kaiser S., Sies H. Lycopene as the most efficient biological carotenoid singlet oxygen quencher. Arch. Biochem. Biophys. 1989;274:532–538. doi: 10.1016/0003-9861(89)90467-0. [DOI] [PubMed] [Google Scholar]
- 7.Rao A.V., Agarwal S. Bioavailability and in vivo antioxidant properties of lycopene from tomato products and their possible role in the prevention of cancer. Nutr. Cancer. 1998;31:199–203. doi: 10.1080/01635589809514703. [DOI] [PubMed] [Google Scholar]
- 8.Tang X., Yang X., Peng Y., Lin J. Protective effects of lycopene against H2O2-induced oxidative injury and apoptosis in human endothelial cells. Cardiovasc. Drug. Ther. 2009;23:439–448. doi: 10.1007/s10557-009-6206-3. [DOI] [PubMed] [Google Scholar]
- 9.Clifford M.N. Diet-derived phenols in plasma and tissue and their implications for health. Planta Med. 2004;70:1103–1114. doi: 10.1055/s-2004-835835. [DOI] [PubMed] [Google Scholar]
- 10.Mehta D., Sharma N., Bansal V., Sangwan R.S., Yadav S.K. Impact of ultrasonication, ultraviolet and atmospheric cold plasma processing on quality parameters of tomato-based beverage in comparison with thermal processing. Innov. Food Sci. Emerg. Technol. 2019;52:343–349. doi: 10.1016/j.ifset.2019.01.015. [DOI] [Google Scholar]
- 11.Lavelli V., Hippeli S., Dornisch K., Peri C., Elstner E.F. Properties of tomato powders as additives for food fortification and stabilization. J. Agric. Food Chem. 2001;49:2037–2042. doi: 10.1021/jf000490e. [DOI] [PubMed] [Google Scholar]
- 12.Story E.N., Kopec R.E., Schwartz S.J., Harris G.K. An update on the health effects of tomato lycopene. Ann. Rev. Food Sci. Technol. 2010;1:189–210. doi: 10.1146/annurev.food.102308.124120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lavelli V., Torresani M.C. Modelling the stability of lycopene-rich by-products of tomato processing. Food Chem. 2011;125:529–535. doi: 10.1016/j.foodchem.2010.09.044. [DOI] [Google Scholar]
- 14.de Souza V.B., Fujita A., Thomazini M., da Silva E.R., Lucon J.F., Genovese M.I., Favaro-Trindade C.S. Functional properties and stability of spray-dried pigments from Bordo grape (Vitis labrusca) winemaking pomace. Food Chem. 2014;164:380–386. doi: 10.1016/j.foodchem.2014.05.049. [DOI] [PubMed] [Google Scholar]
- 15.Goula A.M., Adamopoulos K.G. Effect of maltodextrin addition during spray drying of tomato pulp in dehumidified air: I. Drying kinetics and product recovery. Dry Technol. 2008;26:714–725. doi: 10.1080/07373930802046369. [DOI] [Google Scholar]
- 16.Sramek M., Schweiggert R.M., van Kampen A., Carle R., Kohlus R. Preparation of high-grade powders from tomato paste using a vacuum foam drying method. J. Food Sci. 2015;80:E1755–E1762. doi: 10.1111/1750-3841.12965. [DOI] [PubMed] [Google Scholar]
- 17.Ahmed I., Qazi I.M., Jamal S. Developments in osmotic dehydration technique for the preservation of fruits and vegetables. Innov. Food Sci. Emerg. Technol. 2016;34:29–43. doi: 10.1016/j.ifset.2016.01.003. [DOI] [Google Scholar]
- 18.Correa J.L.G., Ernesto D.B., de Mendonca K.S. Pulsed vacuum osmotic dehydration of tomatoes: Sodium incorporation reduction and kinetics modeling. LWT Food Sci. Technol. 2016;71:17–24. doi: 10.1016/j.lwt.2016.01.046. [DOI] [Google Scholar]
- 19.Lavelli V., Sri Harsha P.S.C., Spigno G. Modelling the stability of maltodextrin-encapsulated grape skin phenolics used as a new ingredient in apple puree. Food Chem. 2016;209:323–331. doi: 10.1016/j.foodchem.2016.04.055. [DOI] [PubMed] [Google Scholar]
- 20.Robert P., Carlsson R.M., Romero N., Masson L. Stability of spray-dried encapsulated carotenoid pigments from rosa mosqueta (Rosa rubiginosa) oleoresin. J. Am. Oil Chem. Soc. 2003;80:1115–1120. doi: 10.1007/s11746-003-0828-4. [DOI] [Google Scholar]
- 21.Souza A.L.R., Hidalgo-Chavez D.W., Pontes S.M., Gomes F.S., Cabral L.M.C., Tonon R.V. Microencapsulation by spray drying of a lycopene-rich tomato concentrate: Characterization and stability. LWT Food Sci. Technol. 2018;91:286–292. doi: 10.1016/j.lwt.2018.01.053. [DOI] [Google Scholar]
- 22.Lavelli V., Kerr W., Harsha P.S.C.S. Phytochemical stability in dried tomato pulp powder and peel as affected by moisture properties. J. Agric. Food Chem. 2013;61:700–707. doi: 10.1021/jf303987v. [DOI] [PubMed] [Google Scholar]
- 23.Lavelli V., Sri Harsha P.S.C., Laureati M., Pagliarini E. Degradation kinetics of encapsulated grape skin phenolics and micronized grape skins in various water activity environments and criteria to develop wide-ranging and tailor-made food applications. Inn. Food Sci. Emerg. Technol. 2017;39:156–164. doi: 10.1016/j.ifset.2016.12.006. [DOI] [Google Scholar]
- 24.Galanakis C.M., editor. Food Waste Recovery: Processing Technologies and Industrial Techniques. Elsevier Inc.; London, UK: 2015. [Google Scholar]
- 25.Xu S., Pegg R.B., Kerr W.L. Effect of processing methods on the quality of tomato products. Food Bioprocess Technol. 2016;9:91–100. doi: 10.1007/s11947-015-1608-7. [DOI] [Google Scholar]
- 26.Boon C.S., McClements D.J., Weiss J., Decker E.A. Role of Iron and Hydroperoxides in the Degradation of Lycopene in Oil-in-Water Emulsions. J. Agric. Food Chem. 2009;57:2993–2998. doi: 10.1021/jf803747j. [DOI] [PubMed] [Google Scholar]
- 27.Chiu Y.T., Chiu C.P., Chien J.T., Ho G.H., Yang J., Chen B.H. Encapsulation of lycopene extract from tomato pulp waste with gelatin and poly(gamma-glutamic acid) as carrier. J. Agric. Food Chem. 2007;55:5123–5130. doi: 10.1021/jf0700069. [DOI] [PubMed] [Google Scholar]
- 28.Xue F., Li C., Liu Y.L., Zhu X.W., Pan S.Y., Wang L.F. Encapsulation of tomato oleoresin with zein prepared from corn gluten meal. J. Food Eng. 2013;119:439–445. doi: 10.1016/j.jfoodeng.2013.06.012. [DOI] [Google Scholar]
- 29.Rocha G.A., Favaro-Trindade C.S., Grosso C.R.F. Microencapsulation of lycopene by spray drying: Characterization, stability and application of microcapsules. Food Bioprod. Process. 2012;90:37–42. doi: 10.1016/j.fbp.2011.01.001. [DOI] [Google Scholar]
- 30.Lavelli V., Kerr W. Apple pomace is a good matrix for phytochemical retention. J. Agric. Food Chem. 2012;60:5660–5666. doi: 10.1021/jf3010993. [DOI] [PubMed] [Google Scholar]
- 31.Basanta M.F., Rojas A.M., Martinefski M.R., Tripodi V.P., De’Nobili M.D., Fissore E.N. Cherry (Prunus avium) phenolic compounds for antioxidant preservation at food interfaces. J. Food Eng. 2018;239:15–25. doi: 10.1016/j.jfoodeng.2018.06.028. [DOI] [Google Scholar]
- 32.Ballesteros L.F., Ramirez M.J., Orrego C.E., Teixeira J.A., Mussatto S.I. Encapsulation of antioxidant phenolic compounds extracted from spent coffee grounds by freeze-drying and spray-drying using different coating materials. Food Chem. 2017;237:623–631. doi: 10.1016/j.foodchem.2017.05.142. [DOI] [PubMed] [Google Scholar]
- 33.Laksmana F.L., Kok P.J.A.H., Frijlink H.W., Vromans H., Maarschalk K.V. Gas permeation related to the moisture sorption in films of glassy hydrophilic polymers. J. Appl. Polym. Sci. 2010;116:3310–3317. doi: 10.1002/app.31854. [DOI] [Google Scholar]
- 34.Prado S.M., Buera M.P., Elizalde B.E. Structural collapse prevents β-carotene loss in a supercooled polymeric matrix. J. Agric. Food Chem. 2006;54:79–85. doi: 10.1021/jf051069z. [DOI] [PubMed] [Google Scholar]
- 35.Harnkarnsujarit N., Charoenrein S., Roos Y.H. Microstructure formation of maltodextrin and sugar matrices in freeze-dried systems. Carbohydr. Polym. 2012;88:734–742. doi: 10.1016/j.carbpol.2012.01.028. [DOI] [Google Scholar]
- 36.Adhikari B., Howes T., Bhandari B.R., Troung V. Effect of addition of maltodextrin on drying kinetics and stickiness of sugar and acid-rich foods during convective drying: Experiments and modelling. J. Food Eng. 2004;62:53–68. doi: 10.1016/S0260-8774(03)00171-7. [DOI] [Google Scholar]
- 37.Sauid S.M., Murthy V.V.P.S. Effect of palm oil on oxygen transfer in a stirred tank bioreactor. J. Appl. Sci. 2010;10:2745–2747. doi: 10.3923/jas.2010.2745.2747. [DOI] [Google Scholar]
- 38.Cuvelier M.E., Soto P., Courtois F., Broyart B., Bonazzi C. Oxygen solubility measured in aqueous or oily media by a method using a non-invasive sensor. Food Control. 2017;73:1466–1473. doi: 10.1016/j.foodcont.2016.11.008. [DOI] [Google Scholar]
- 39.Yeum K.J., Russell R.M., Krinsky N.I., Aldini G. Biomarkers of antioxidant capacity in the hydrophilic and lipophilic compartments of human plasma. Arch. Biochem. Biophys. 2004;430:97–103. doi: 10.1016/j.abb.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 40.Marinova E.M., Toneva A., Yanishlieva N. Comparison of the antioxidative properties of caffeic and chlorogenic acids. Food Chem. 2009;114:1498–1502. doi: 10.1016/j.foodchem.2008.11.045. [DOI] [Google Scholar]
- 41.AOAC . Official Methods of Analysis. 15th ed. Association of Official Analytical Chemists; Washington, DC, USA: 1990. [Google Scholar]