Table 2.
Et3N-catalyzed synthesis of 2,3,6,7,9-pentaazabicyclo[3.3.1]nona-3,7-dienes 2a–p.
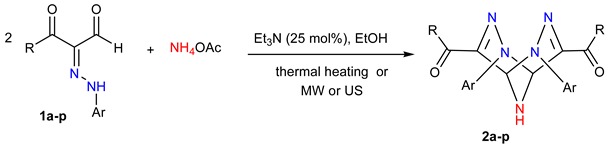
| Run | Products | R | Ar | Conv. Heating a | Sonication a | MW a Irradiation | |||
|---|---|---|---|---|---|---|---|---|---|
| Yield b% | Time (h) | Yield b % | Time (min) | Yield b % | Time (min) | ||||
| 1 | 2a | 4-FC6H4 | 4-ClC6H4 | 78 | 3 | 89 | 50 | 94 | 3 |
| 2 | 2b | 4-FC6H4 | 4-BrC6H4 | 77 | 4 | 87 | 60 | 93 | 4 |
| 3 | 2c | C6H5 | 4-ClC6H4 | 70 | 3 | 81 | 50 | 89 | 4 |
| 4 | 2d | C6H5 | 4-BrC6H4 | 72 | 5 | 82 | 70 | 90 | 6 |
| 5 | 2e | C6H5 | 2-NO2C6H4 | 68 | 4 | 80 | 70 | 86 | 5 |
| 6 | 2f | 4-ClC6H4 | C6H5 | 71 | 3 | 83 | 40 | 90 | 3 |
| 7 | 2g | 4-ClC6H4 | 4-BrC6H4 | 73 | 5 | 84 | 70 | 91 | 5 |
| 8 | 2h | 4-BrC6H4 | 4-ClC6H4 | 86 | 4 | 87 | 70 | 92 | 5 |
| 9 | 2i | 4-BrC6H4 | 4-BrC6H4 | 84 | 5 | 86 | 80 | 92 | 6 |
| 10 | 2j | 4-OMeC6H4 | C6H5 | 66 | 5 | 77 | 70 | 82 | 5 |
| 11 | 2k | 4-OMeC6H4 | 4-ClC6H4 | 65 | 6 | 77 | 90 | 81 | 6 |
| 12 | 2l | 4-OMeC6H4 | 4-BrC6H4 | 67 | 8 | 78 | 100 | 83 | 7 |
| 13 | 2m | 4-NO2C6H4 | 4-ClC6H4 | 67 | 7 | 79 | 110 | 84 | 7 |
| 14 | 2n | 4-NO2C6H4 | 4-BrC6H4 | 71 | 8 | 80 | 100 | 86 | 8 |
| 15 | 2o | CH3 | 4-ClC6H4 | 62 | 7 | 73 | 90 | 81 | 8 |
| 16 | 2p | CH3 | 4-BrC6H4 | 64 | 6 | 75 | 110 | 81 | 9 |
a Reaction conditions: 3-Oxo-2-arylhydrazonopropanals 1a–p (5 mmol), ammonium acetate (10 mmol) and Et3N (25 mol%) in EtOH (7 mL) at reflux temperature for conventional heating 3~8 h, ultrasonic irradiation at 80 °C (110 W) for 50~110 min, or microwave irradiation at 80 °C (200 W) for 3~9 min. b Isolated yields. Conv. = conventional, MW = microwave.
