Abstract
The National Institutes of Health and the Association of Zoos and Aquariums recommend that captive chimpanzees be housed in multi-male, multi-female, age-diverse groups of no less than seven individuals. These recommendations are rooted in the idea that captive chimpanzee groups should be modeled after free-ranging, wild, fission-fusion chimpanzee societies. However, captive chimpanzees do not face the environmental pressures faced by wild chimpanzees, including food scarcity, inter-group competition, and predation. As such, it has been posited that wild, natural conditions may not be the most relevant metric for defining optimal captive chimpanzee group sizes and compositions. Additionally, captive housing poses a set of restrictions on group sizes and compositions, including the need to balance large, multi-male groups with space per animal limitations and intra-group aggression. In the present study, we examined the behavioral effects of group size, within-group age range, and percentage of males in the group. We collected 713 hr of focal animal samples across 120 captive chimpanzees housed in social groups of 4–10 individuals using a 58-behavior ethogram. Chimpanzees housed in groups with a large age range exhibited less inactivity and more locomotion than chimpanzees housed in groups with smaller age ranges. Additionally, chimpanzees in groups of ≥7 with less than half males showed the highest levels of locomotion. Lastly, chimpanzees in groups of ≥7 with at least half males showed the highest levels of affiliation. There were no other significant differences in behavior as a function of these variables or their interactions. These findings lend some support to the existing group size and composition recommendations, providing empirical evidence that there may be certain advantages to housing captive chimpanzees in larger groups with a more diverse age range and/or more males. These results also have practical implications for behavioral management programs across captive settings.
Keywords: behavior, captivity, chimpanzee, group composition, group size, management
1 |. INTRODUCTION
Several agencies have recently put forth various recommendations regarding captive chimpanzee housing and management. The National Institutes of Health (NIH) recommend that captive chimpanzees be maintained in Ethologically Appropriate Environments (EAEs), defined as captive environments that promote all natural behaviors exhibited by wild, free-ranging chimpanzees (NIH, 2013). The NIH states that these wild chimpanzee societies and environments should serve as the “gold standard” after which the captive environment should be modeled (NIH, 2013). Therefore, to provide the social complexity and stimulation necessary for such cognitively complex animals, chimpanzees should be maintained in multi-male, multi-female groups of at least seven animals per social group (NIH, 2013). Furthermore, the Chimpanzee Care Manual put forth by the Association of Zoos and Aquariums (AZA, 2010) states that chimpanzees should be housed in groups with age and sex diversity, with at least three males and five females per group. These recommendations are based on the idea that modeling the group sizes and compositions of wild chimpanzees enhances the well-being of captive chimpanzees (AZA, 2010; Else, 2013; NIH, 2013). Similarly, many suggest that natural conditions and behaviors can be simulated through functionally appropriate captive environments (FACEs), which promote opportunities to engage in species-typical behavior through functional simulations of natural conditions (Bettinger, Leighty, Daneault, Richards, & Bielitzki, 2017; Bloomsmith, Hasenau, & Bohm, 2017; Dye, 2017, Honess, 2017; Reamer, Haller, Lambeth, & Schapiro, 2017; Schapiro, 2017; Williams & Ross, 2017). These complimentary concepts can be used to investigate and implement optimal captive group sizes and compositions for chimpanzees
The recommendations for captive chimpanzee group size and composition are based primarily on data gathered from wild chimpanzees that live in large, fission-fusion societies of up to 120 individuals per community (Nishida, 1968), with an average party size of 5.7 individuals (Table 1; Wrangham, 1992). However, wild chimpanzees live in such social systems because they must adapt to the environmental pressures present in their immediate environment (Humle, 2003; Symington, 1990), including food and water scarcity and threats from predators (both human and non-human) and conspecifics (Boesch & Boesch-Achermann, 2000; Else, 2013; Goodall, 1986; Lehmann & Boesch, 2004; Price & Stoinski, 2007; Sugiyama, 2004; Wrangham, 1992). For example, chimpanzees tend to aggregate in smaller parties when they must travel longer distances for access to resources (Chapman et al., 1995), during times of low fruit availability, and when there are fewer estrous females (Boesch & Boesch-Achermann, 2000). Interestingly, when environmental pressures are removed from wild chimpanzee environments, group size seems to decrease and stabilize. In Bossou, Guinea, during a time when there was high year-round food availability, restricted range size, and no predators or inter-group competition, group size remained relatively low and stable at around 20 chimpanzees (Boesch & Boesch-Achermann, 2000). Furthermore, small chimpanzee communities (around 20) at Taï Forest, Cote d’Ivoire and Bossou, Guinea have been found to be more cohesive and stable with a lowered occurrence of fission-fusion behavior (Boesch, 1996; Boesch & Boesch-Achermann, 2000; Lehmann & Boesch, 2004; Sugiyama, 2004). Else (2013) reasons that findings like these may bring into question the relevance of fission-fusion behavior for captive chimpanzee groups, all of which may be considered “small communities,” given their group size and lower number of males per group. Since captive chimpanzees do not face most of the environmental pressures experienced by chimpanzees in the wild (e.g., captive chimpanzees are provisioned food daily, and experience no predation or competition for food; Else (2013); Price & Stoinski (2007)), natural, wild conditions may not be the most relevant metric for defining optimal captive group size and composition (Reamer et al., 2017).
TABLE 1.
Wild chimpanzee party sizes across Africa
| Average party size | Study site | Citation |
|---|---|---|
| 5.7 per party (range from 4 to 8.3 per party) | Across Africa: Assirik; Gombe; Mahale; Kibale (Ngogo); & Bossou, Guinea | Boesch (1996) |
| 5 per party | Kibale National Park, Uganda | Chapman, Chapman, and Wrangham (1995) |
| 4–10 per party | Taï National Park, Côte d’Iviore | Boesch and Boesch-Achermann (2000) |
| Less than 10 per party | Across Africa: Gombe; Mahale; Kibale (Ngogo); & Bossou, Guinea | Wrangham (1992) |
| 1–5 per party | Mahale Mountains National Park, Tanzania | Matsumoto-Oda, Hosaka, Huffman, and Kawanaka (1998) |
| 2.5 per party in larger community | Taï National Park, Côte d’Iviore | Lehmann and Boesch (2004) |
| 7 per party in smaller community | ||
| 19 per party when travelinga | Mt. Assirik, Senegal | Tutin, McGrew, and Baldwin (1983) |
| 5 per party when socializinga | ||
| 8 per party when traveling | Mahale Mountains National Park, Tanzania | Boesch and Boesch-Achermann (2000); Watts and Mitani (2001) |
| 20 per party when hunting |
Median party size shown.
Captive housing poses a set of restrictions that must be balanced with optimal captive chimpanzee group size and composition, including physical facility limitations and management of species-typical aggression (Bloomsmith & Baker, 2001; Else, 2013; Morgan & Tromborg, 2007; Price & Stoinski, 2007). Despite these restrictions, captive chimpanzees have been successfully maintained in social groups of various sizes and compositions, including multi-male, multi-female groups of up to 20 individuals in various laboratory settings (Alford, Bloomsmith, Keeling, & Beck, 1995; Bloomsmith & Baker, 2001; Riddle et al., 1982), and groups of up to 25 in chimpanzee sanctuaries (NIH, 2013). Additionally, captive chimpanzees have been successfully housed in all-male, or “bachelor,” groups (Alford et al., 1995; Bloomsmith & Baker, 2001), although all-male groups tend to exhibit higher levels of intra-group aggression than mixed-sex groups (Fritz & Howell, 1999). Within mixed-sex groups, few differences in behavior have been found between uni-male and multi-male groups, although males do seem to prefer other males as social partners (Bloomsmith & Baker, 2001), and rates of wounding seem to be higher in uni-male groups than multi-male groups (Ross, Bloomsmith, Bettinger, & Wagner, 2009). Groups with a higher number of males seem to exhibit higher incidences of aggression (Alford et al., 1995), and larger groups (19–21 individuals) seem to sustain higher numbers of minor wounds than smaller groups (7–12 individuals) (Baker, Seres, Aureli, & de Waal, 2000). Pruetz and McGrew (2001) posit that, since large, stable groups are not common in the wild (but see Boesch & Boesch-Achermann, 2000), a group size threshold might exist, such that there is a limit on the number of chimpanzees that can live in constant, amicable presence of one another. Indeed, captive chimpanzees exhibit coping strategies in response to both short-and long-term crowding conditions, including conflict-avoidance and tension-reduction strategies, respectively (de Waal, Aureli, & Judge, 2000; Nieuwenhuijsen & de Waal, 1982; Videan & Fritz, 2007).
Thus far, only one study has empirically examined the NIH’s (2013) group size recommendation. Reamer et al. (2016) found that chimpanzees housed in groups of seven or more exhibited higher levels of affiliative and abnormal behavior compared to groups of six or fewer. As part of a larger project that examines the behavioral effects of captive environments and refinements to behavioral management techniques (Neal Webb, Hau, & Schapiro, 2018a, 2018b), the current study aimed to empirically examine group size and group composition recommendations to provide additional data for use in defining captive chimpanzee FACEs. Specifically, we explored whether the behavior of captive chimpanzees housed in groups ranging from 4 to 10 animals differed as a function of 1) group size; 2) the percentage of males within the group; 3) within-group age range; and 4) interactions between these variables. If larger groups with more males and a larger age range are more functionally appropriate for captive chimpanzees, chimpanzees in such groups should exhibit higher levels of welfare-related behaviors (e.g., higher levels of affiliation, proximity to group mates, behavioral diversity), and lower levels of behaviors indicative of poor well-being (e.g., abnormal behavior, rough-scratching, and aggression). However, it is possible that the relationship between group size, composition, and welfare is not so simple. Based on findings from previous studies (Baker et al., 2014; Pruetz & McGrew, 2001; Reamer et al., 2016), we predicted that chimpanzees would exhibit several behavioral differences across varying group sizes and compositions, some with potentially positive, and some with potentially negative, welfare consequences. Specifically, we predicted that chimpanzees in larger groups with more males and a larger age range would exhibit increased behavioral diversity and locomotion, lower inactivity, and closer proximity to group mates, likely indicative of enhanced welfare. Furthermore, since larger group size and more males may increase within-group tension due to a higher probability of aggression (Alford et al., 1995; Baker et al., 2000; de Waal et al., 2000; Fritz & Howell, 1999; Nieuwenhuijsen & de Waal, 1982; Videan & Fritz, 2007), we predicted that chimpanzees in larger groups with more males would exhibit more rough scratching, aggressive, and affiliative behavior, which may indicate potential decreases in welfare. The pattern of behavioral differences should be interpreted as a whole to determine the effects of group size and composition on welfare.
2 |. METHODS
2.1 |. Subjects and housing
Subjects included 120 captive chimpanzees at the Michale E. Keeling Center for Comparative Medicine and Research (KCCMR) of The University of Texas MD Anderson Cancer Center. At the end of data collection, chimpanzees lived in 17 separate social groups of 4–10 animals per group (Table 2). The number of individuals per group occasionally changed throughout the course of the study, as introductions between groups and/or of individual animals occurred due to veterinary or behavioral management issues. Subjects included 73 females and 47 males ranging in age from 15 to 56 (M = 31.39 years). Of the 120 chimpanzees, 78 were mother-reared, 27 were nursery-reared, and 15 were wild-born with an unknown rearing history.
TABLE 2.
Group size and composition at the end of the study period
| Group | Group size | % Male | Age range (yrs) | Average age (yrs) |
|---|---|---|---|---|
| D4 | 4 | 30 | 3 | 49 |
| D2 | 5 | 0 | 20 | 45 |
| D3 | 5 | 33 | 19 | 36 |
| D8 | 5 | 88 | 21 | 31 |
| D1 | 6 | 17 | 11 | 34 |
| D7 | 6 | 39 | 21 | 30 |
| C4 | 7 | 29 | 37 | 35 |
| C6 | 7 | 39 | 6 | 33 |
| DQ | 7 | 29 | 15 | 28 |
| C1 | 8 | 50 | 15 | 30 |
| C2 | 8 | 50 | 27 | 29 |
| C3 | 8 | 50 | 8 | 31 |
| C7 | 8 | 50 | 25 | 33 |
| C8 | 8 | 50 | 20 | 45 |
| CA | 9 | 44 | 12 | 26 |
| CB | 9 | 54 | 5 | 26 |
| C5 | 10 | 39 | 6 | 34 |
Group size and composition for some groups changed during the course of this study due to behavioral management and veterinary reasons. Therefore, the numbers presented in this table represent averages across the study period and are rounded to the nearest whole number.
The KCCMR facility is accredited by AAALAC International, demonstrating that housing and care meet or exceed all current USDA guidelines. Groups were housed in indoor-outdoor corrals or Primadomes™ (Neal Webb, Hau, & Schapiro, 2018a) with access to all areas day and night. The research conducted in this study complied with the approved protocols of the UTMDACC Institutional Animal Care and Use Committee, and adhered to the legal requirements of the United States and to the American Society of Primatologists’ Principles for the Ethical Treatment of Primates.
2.2 |. Procedure
We used 15-min focal animal samples for behavioral observations (Altmann, 1974). Each chimpanzee served as a focal animal in a minimum of 22 focal observations, for a total of 713 hr of observation. Observations were recorded on a laptop computer using Noldus Observer XT 10 (2010) software between 0700 and 1600 from August 2016 through May 2018. Categories of behavior included locomotive, aggressive, submissive, object manipulation, self-directed, abnormal, affiliative, inactive, and other. Proximity of the focal animal to other animals within the group (touching, near, distant) was also recorded (see Supplemental Materials for ethogram). Since data were collected regardless of introductions between groups and individuals that occurred during the course of the study, the number of individuals per group was noted during each focal observation. The mean number of chimpanzees in each group was then calculated at the end of the study by averaging the number of animals in the group across all focal observations for each chimpanzee.
2.3 |. Data analysis
Total durations of behavior were averaged across all observations for each subject. Average durations of “out-of-view” were subtracted from total possible observation time (900 s) to create average “in-view” durations for the denominator (chimpanzees were “in-view” for an average of 98% of each observation). Average durations of each behavior were then converted into percentage of time [(average behavior duration in seconds/”in-view duration” in seconds) *100] for each chimpanzee. Average age of each group, within-group age range (= oldest chimpanzee age − youngest chimpanzee age), and percentage of males within each group were also calculated. A proximity score [percentage of time spent (near + touching)/total in-view duration] was calculated to represent the percentage of time spent touching or in close proximity to group mates. Lastly, we calculated a behavioral diversity score that served as a measure of the total number of different behaviors (excluding negative welfare-related behaviors) exhibited by each chimpanzee throughout the course of the study. This was calculated by counting the number of behaviors exhibited across all observations (i.e., all positive welfare-related behaviors that had a non-zero duration) for each chimpanzee.
With the exception of three behaviors (inactive, self-groom, and behavioral diversity), all data were positively skewed. The predictor variables were categorized to create, insofar as possible, equal intervals between categories, as well as relatively equal numbers of chimpanzees within categories of the variable. Group size and percentage of males were dichotomized (≤6 and ≥7; ≤49% and ≥50%, respectively). Age range was categorized into three levels: small range (≤9 years), medium range (10–19 years), and large range (≥20 years). Since smaller groups tend to be housed in Primadomes® and larger groups in corrals, we used enclosure type as a covariate. Average age of the group was used as a continuous covariate. Group average age and within-group age range were not correlated, r = 0.022, p = 0.815. We chose to use average age of the group as a covariate rather than an independent variable for several reasons. First, previous research shows that older age affects behavior; specifically, older chimpanzees exhibit decreased mobility, lower levels of aggression, locomotion, and object manipulation than younger chimpanzees (Baker, 2000; Magden et al., 2013; Nunamaker, Lee, & Lammey, 2012). Second, the NIH and AZA make no recommendations relating to group average age, other than stating that chimpanzees should be housed in “age diverse” groups, which we believe is better represented by age range. Lastly, our sample size limited the number of independent variables that could be included in the statistical model while still allowing assessment of interactions between variables (Hair, Black, Babin, Anderson, & Tatham, 2010).
We used a bootstrapped MANCOVA to assess the effects of group size, within-group age range, and percentage of males within the group on behavioral diversity scores, proximity scores, rough-scratching, aggressive, locomotive, affiliative, abnormal, and inactive behaviors. Since MANCOVAs adjust for experiment-wide error rate (Hair et al., 2010; Nakagawa, 2004; Perneger, 1998), we used p ≤ 0.05 for significant differences and 0.06 ≤ p ≤ 0.07 for trending differences. For significant omnibus F tests, we used Bonferroni-corrected post-hoc comparisons for main effects. We were also interested in interaction effects between group size and percentage of males, and group size and age range. Unfortunately, our sample size was not adequate to assess the three-way interaction among all three independent variables (Hair et al., 2010). Simple effects tests were used for further investigation of interaction effects, including independent samples t-tests for group size and percentage of males, and one-way ANOVAs with Bonferroni post-hoc tests for age range. Estimated marginal means and standard errors are reported. All analyses were conducted using IBM SPSS Statistics 24 (IBM Corporation, Chicago, IL).
3 |. RESULTS
The MANCOVA showed a significant effect of within-group average age as a covariate on two behaviors, including affiliative behaviors ([F (1,107) = 6.35, p = 0.013], and inactivity [F(1,107) = 4.96, p = 0.028]. There were no significant or trending effects of housing type (dome vs. corral) as a covariate (p > 0.10).
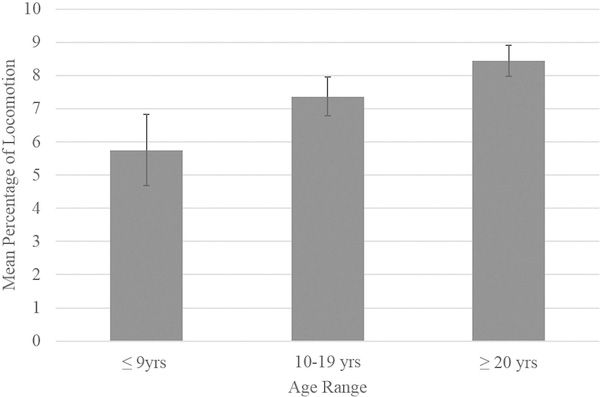
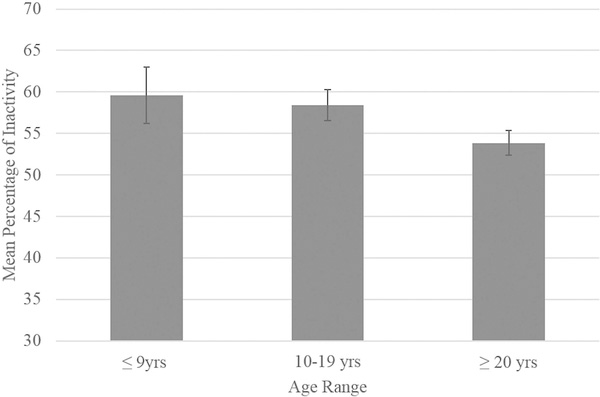
There were no significant main effects of group size on behavior (p > 0.10). There were also no significant main effects of percentage of males on any of the dependent variables (p > 0.10). There was a trending main effect of within-group age range on locomotive behavior [F(2,107) = 2.77, p = 0.067]. Post-hoc comparisons failed to reach significance (small age range ≤9 years: M = 5.75, SE = 1.07; medium age range 10–19 years: M = 7.37, SE = 0.59; large age range ≥20 years: M = 8.44, SE = 0.47; p > 0.10; see Figure 1). There was a significant main effect of age range on inactivity [F(2,107) = 3.11, p = 0.049]. Chimpanzees housed in groups with a large age range exhibited the lowest percentage of inactivity (M = 53.86, SE = 1.49), followed by chimpanzees in groups with a medium age range (M = 58.45, SE = 1.88), and those in groups with a small age range (M = 59.63, SE = 3.40), although none of these post-hoc comparisons reached statistical significance (p > 0.10; see Figure 2). There were no other significant main effects of age range on the remaining behaviors, including proximity and behavioral diversity scores, rough scratching, aggressive, abnormal, and affiliative behaviors (p > 0.10).
FIGURE 1.
Mean percentage of locomotion as a function of within-group age range category. Error bars represent standard error of the mean
FIGURE 2.
Mean percentage of inactivity as a function of withingroup age range category. Error bars represent standard error of the mean
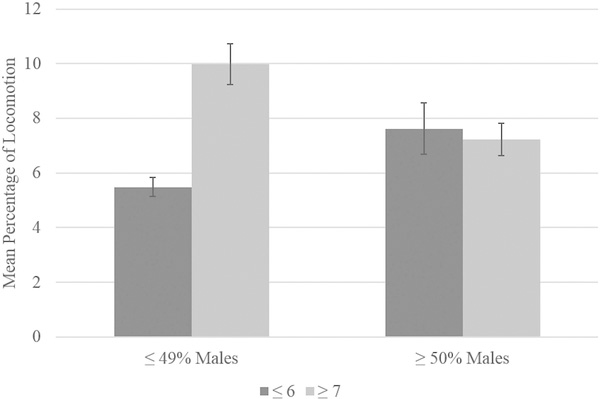
Regarding interaction effects, there was a significant interaction between group size and percentage of males within a group for locomotion, F(1,107) = 6.68, p = 0.011 (see Figure 3). Simple effects tests showed that chimpanzees in groups of six or fewer with less than 50% males showed a significantly lower percentage of locomotion (M = 5.49, SE = 0.35) than chimpanzees in groups of six or fewer, but with at least 50% males [M = 7.62, SE = 0.93; t(53) = −2.41, p = 0.02], whereas those in groups of seven or more with less than 50% males showed a higher percentage of locomotion (M = 9.98, SE = 0.79) than chimpanzees in groups of seven or more, but with at least 50% males [M = 7.22, SE = 0.59; t(63) = 2.89, p = 0.005]. Furthermore, among chimpanzees housed in groups with less than 50% males, chimpanzees in groups of six or fewer showed significantly less locomotion (M = 5.49, SE = 0.35) than chimpanzees in groups of seven or more [M = 9.98, SE = 0.74; t(69) = −6.23, p = 0.0001]. There was no significant difference in mean percentage of locomotion between chimpanzees housed in groups of six or fewer (M = 7.62, SE = 0.93) and seven or more (M = 7.22, SE = 0.59) when they were housed in groups with 50% or more males (p > 0.10).
FIGURE 3.
Locomotion interaction effect between group size and percentage of males within the group. Error bars represent standard error of the mean
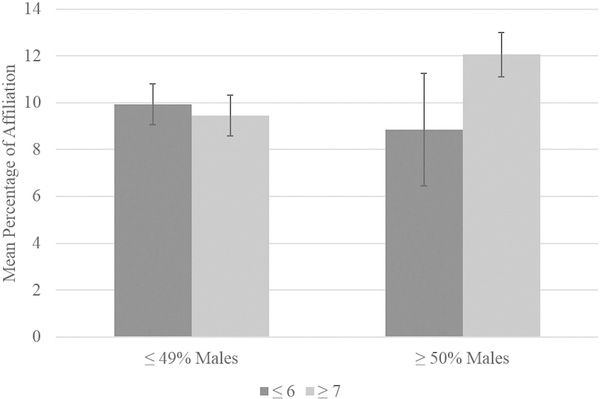
There was also a significant interaction effect between group size and percentage of males for affiliative behavior, F(1,107) = 4.92, p = 0.029 (see Figure 4). Simple effects tests showed that, among chimpanzees housed in groups of seven or more, those also housed in groups with at least 50% males showed a higher percentage of affiliative behavior (M = 12.06, SE = 0.95) than those in groups with less than 50% males (M = 9.45, SE = 0.88), although this difference was trending (p = 0.067). There were no significant simple effects in affiliative behavior between chimpanzees housed in groups of six or fewer with less than 50% males (M = 9.93, SE = 0.88) compared to at least 50% males (M = 8.86, SE = 2.41), or compared to those housed in groups of seven or more (M = 9.45, SE = 0.88; p > 0.01). The simple effect between chimpanzees housed in groups with at least 50% males with six or fewer animals (M = 8.86, SE = 2.41) and those in groups with at least 50% males with seven or more animals (M = 12.06, SE = 0.95) was also not significant (p > 0.10).
FIGURE 4.
Affiliation interaction between group size and percentage of males within the group. Error bars represent standard error of the mean
4 |. DISCUSSION
The current study empirically examined existing recommendations pertaining to group size and group composition factors. The results provide partial support for these recommendations, in that locomotion, inactivity, and affiliative behavior differed as a function of main effects of, and/or interaction effects between, group size, within-group age range, and percentage of males in the group. Specifically, chimpanzees in groups with a large age range showed less inactivity and more locomotion than chimpanzees in groups with a small or medium age range. Furthermore, chimpanzees in groups of seven or more with less than half males exhibited the highest percentage of locomotion, and chimpanzees in groups of seven or more with at least half males showed the highest level of affiliative behavior. Taken together, these data suggest that there are certain advantages to housing captive chimpanzees in larger, more age-diverse groups with more males. However, as discussed below, there is some mixed evidence regarding the welfare implications of these behavioral changes. Additionally, it is important to interpret the data in the context of behavioral management implications, including constraints of captive housing and the needs of individual chimpanzees. From a behavioral management perspective, data from the current study also suggest that chimpanzees in smaller groups with fewer males and/or a smaller age range do not experience diminished well-being as a result of this social housing. As such, these results suggest that chimpanzees experience certain benefits to welfare when housed in larger groups with larger age ranges and/or more males, but, given the constraints of captive housing and the needs of individual chimpanzees, different combinations of group sizes and composition can be implemented to promote well-being.
Locomotive behavior differed as a function of within-group age range and an interaction between group size and percentage of males within the group. Although our sample size limited our ability to conduct a three-way interaction among these independent variables, the data showed that chimpanzees exhibited the highest level of locomotion in larger groups with less than half males. Additionally, locomotion increased as within-group age range increased. Higher locomotion in chimpanzees is generally considered to be indicative of positive welfare, whereas decreases in locomotion are thought to be indicative of decreased well-being (Baker et al., 2014; Pruetz & McGrew, 2001). This is partly because chimpanzees in the wild exhibit high levels of locomotion (∼15–25%: Doran, 1997; Boesch & Boesch-Achermann, 2000; Pruetz & McGrew, 2001; Yamanashi & Hayashi, 2011), and the goal is to simulate the behavior of wild counterparts (NIH, 2013; Else, 2013; Pruetz & McGrew, 2001; Yamanashi & Hayashi, 2011). However, wild chimpanzees may exhibit high levels of locomotion out of necessity, to obtain access to food and water and in defense of territory (Boesch & Boesch-Achermann, 2000), and it is unclear whether captive chimpanzees prefer to spend the same amount of time locomoting as their wild counterparts when given the choice. Although the assumption is that higher locomotion is indicative of enhanced well-being, the relationship between locomotion and welfare may not be so simple (Baker, 1996, 1997, 2000, 2004; Duncan, Jones, van Lierop, & Pillay, 2013; Howell, Schwandt, Fritz, Roeder, & Nelson, 2003; Neal Webb, Hau, & Schapiro, 2018b;Ross, Wagner, Schapiro,& Hau, 2010). Increased locomotion has been used as an indicator of tension and anxiety in other species of NHP, including both new and old world monkeys (Dufour, Sueur, Whiten, Buchanan-Smith, 2011; Engel et al., 2009; Erickson et al., 2005; Meyer, Novak, Bowman, & Harlow, 1975; Schino, Perretta, Taglioni, Monaco, & Troisi, 1996), as well as apes. For example, gorillas exhibited increased locomotion (as well as increased self-directed behaviors, aggression, and prosocial behaviors) in response to increased social density (Ross et al., 2010). In chimpanzees, increases in locomotion under certain circumstances (e.g., single housing) have been suggested to be indicative of tension and anxiety (Baker, 1996), and decreases in locomotion have been suggested to be indicative of a calmer state and enhanced well-being (Howell et al., 2003). If we maintain the assumption that higher levels of locomotion are indicative of enhanced well-being, this particular finding may provide support for the NIH’s group size recommendation (NIH, 2013) and the AZA’s age-diversity recommendation (2010). However, given the complexities of the relationship between locomotion and well-being, we are hesitant to explicitly state that the increased locomotion found in the current study is a positive finding. Importantly, this highlights the need for additional empirical evaluations of locomotion as an indicator of well-being.
Affiliative behavior differed as a function of the interaction between group size and percentage of males, as chimpanzees housed in groups of seven or more with at least 50% males showed the highest level of affiliative behavior. This is consistent with Reamer et al. (2016), which found increased affiliative behavior in groups of seven or more chimpanzees. It may be that larger groups with more males exhibit higher levels of affiliative behavior as a way to decrease tension within the group. Previous studies have found that larger groups and groups with more males tend to exhibit a higher incidence of aggression (Alford et al., 1995; Baker et al., 2000; Fritz and Howell, 1999). Additionally, some studies have found that chimpanzees employ a tension-reduction strategy in response to certain social conditions, such that affiliation increases, but aggression does not (de Waal et al., 2000; Judge & de Waal, 1997). The increased affiliation is thought to decrease the tension within the group, which then lowers chances of agonism (de Waal et al., 2000; Judge & de Waal, 1997; Videan & Fritz, 2007; but see Aureli & de Waal, 1997). It is possible that a similar strategy was adopted by chimpanzees in the present study. Chimpanzees in larger groups with more males exhibited higher levels of affiliation (an increase of approximately 2.5%), but not aggression, compared to smaller groups with a lower percentage of males. This suggests that increased levels of affiliative behavior may prevent aggression and maintain accord within the group. Another, perhaps more parsimonious, explanation is that the increased affiliation in larger groups with more males is indicative of enhanced well-being (Muroyama & Sugiyama, 1994) as a result of a more functionally appropriate captive (social) environment. Given that males prefer other males as grooming partners (Bloomsmith & Baker, 2001), it is possible that the increased affiliation may be explained by increased grooming (and other affiliative) opportunities between males.
Behavioral management continually evolves toward optimal captive care. In the context of functionally appropriate group sizes and compositions, and in keeping with the assumption that higher levels of locomotion and affiliation are indicative of heightened well-being, there may be some room for welfare enhancement by increasing locomotion and affiliation, and decreasing inactivity through increases in social complexity. The lower percentage of inactivity and higher percentage of locomotion in groups with a wider age range, as well as the higher percentage of affiliation in larger groups with more males may be an indication that larger, more age-diverse groups with more males possess higher social complexity (AZA, 2010; Bloomsmith & Baker, 2001; Pruetz & McGrew, 2001). This complexity may require increased locomotion and affiliation (and decreased inactivity) in order to maintain social bonds, as chimpanzees move between and among groupmates to socialize. If this is the case, these results may indicate that larger, more age-diverse groups with more males are more functionally appropriate for captive chimpanzees. As such, these findings could be applied in a variety of captive settings. For example, careful construction of group size and composition could be one way to ensure a higher levels of locomotive behavior within a group, thereby reducing chances of obesity in the long-term (Bridges, Mocarski, Reamer, Lambeth, & Schapiro, 2013; Pruetz & McGrew, 2001). Behavioral managers attempting to create new social groups may choose animals based on age to create a wider age range, or may include more males in a group (in addition to a host of other compatibility factors) to increase affiliation and locomotion, and reduce inactivity. However, these data may be limited in their applications to existing social groups, as there are likely easier and safer ways to increase locomotion than by manipulating group size, age range, and percentage of males within an already-established social group, including rotation between different enclosure types and increasing novelty and complexity within the environment (Bloomsmith & Baker, 2001; Coe, Fulk, & Brent, 2001; Lukas, Hoff, & Maple, 2003; Neal Webb, Hau, & Schapiro, 2018a). Furthermore, as the captive population of NIH-owned and NIH-supported chimpanzees continues to age, and the age distribution becomes more skewed, behavioral management teams will have limited options in forming social groups based on age range.
Captive housing poses certain constraints on group size and composition (Bloomsmith & Baker, 2001; Else, 2013; Morgan & Tromborg, 2007; Price & Stoinski, 2007), and behavioral managers must work within these constraints when attempting to optimize social housing. Various issues must be accounted for in the discussion, implementation, and evaluation of appropriate captive social groups, including limited space, increased risk of aggression with more males, an aging population that requires increased medical care and prevents large within-group age ranges, and compatibility of certain individuals with health- or management-related problems (Baker et al., 2000; Bloomsmith & Baker, 2001; Bridges et al., 2015; Hopkins & Latzman, 2017; Reamer et al., 2017). Overall, it may not be feasible to house all chimpanzees in large groups with a wide age range and/or more males. In this regard, data from the current study as a whole seem to be consistent with the explanation that larger group sizes with more males and wider age ranges (perhaps as a function of increases in social complexity) provide an increase in the functional appropriateness of the captive environment. However, this does not imply that other group sizes and compositions (e.g., groups of six or fewer with one to two males and a smaller age range) are NOT functionally appropriate. Levels of aggression, abnormal behaviors, and rough scratching (commonly used indicators of decreased well-being) were not significantly different across groups, and levels of each of these behaviors were low across all group sizes and compositions, comprising less than one-half of one percent, one percent, and one-third of one percent of all activity, respectively. Lastly, the overall levels of affiliation, inactivity, and locomotion across group sizes and compositions, while statistically different, were all within ranges reported in other studies of chimpanzee behavior (Baker, 2000, 2004; Duncan et al., 2013; Ross et al., 2010), and are similar to ranges reported in the wild (Pruetz & McGrew, 2001; Yamanashi & Hayashi, 2011). Therefore, chimpanzees in smaller groups, and/or with a smaller age range, and/or with fewer males do not seem to be suffering decreases in, or detriments to, welfare. We would suggest that it is beneficial to house chimpanzees in larger groups with a wider age range and/or more males. However, chimpanzees that must be housed in other group sizes and compositions due to health- or management-related reasons can still be housed in functionally appropriate social environments, and can still experience levels of well-being comparable to their counterparts in larger groups with more males and a larger age range.
This study demonstrates the importance of empirical examinations of existing recommendations. The higher levels of locomotion and affiliation observed in larger groups with a wider age range and/or more males are positive findings indicative of enhanced well-being in the present study. From an applied behavioral management perspective, these data also suggest that chimpanzees in various group sizes and compositions may experience comparable welfare, an important fact given the limits that captive housing puts on group size and composition. There were no behavioral differences found as a function of group size alone or percentage of males alone, underscoring the importance of examining multiple factors that interact to affect behavior and welfare. Additionally, the group sizes and compositions found in the current study are similar to party sizes and compositions of wild chimpanzees (Else, 2013; see Table 1), but do not necessarily replicate the fission-fusion society of wild chimpanzees. This highlights the importance of functional simulations within the captive environment (Bloomsmith et al., 2017; Else, 2013; Reamer et al., 2017). However, more data are needed to examine 1) behavioral effects of group sizes larger than 10 and 2) the ways in which group composition factors may interact with larger group sizes to affect behavior.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank Susan (Lambeth) Pavonetti, Dr. Michele Mulholland, Lisa Reamer, Mary Catherine Mareno, and Dr. Gill Vale for helpful comments during preparation of this paper. We would also like to thank the carestaff at the National Center for Chimpanzee Care for care of the chimpanzees. This work was supported by NIH U42-OD 011197 and the University of Copenhagen.
Funding information
Det Sundhedsvidenskabelige Fakultet, Københavns Universitet; National Institutes of Health, Grant number: U42-OD 011197
Footnotes
CONFLICTS OF INTEREST
We have no conflicts of interest to declare.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
REFERENCES
- Alford PL, Bloomsmith MA, Keeling ME, & Beck TF (1995). Wounding aggression during the formation and maintenance of captive, multimale chimpanzee groups. Zoo Biology, 14(4), 347–359. 10.1002/zoo.1430140406 [DOI] [Google Scholar]
- Altmann J (1974). Observational study of behavior: Sampling methods. Behaviour, 49, 227–267. 10.1163/156853974(00534 [DOI] [PubMed] [Google Scholar]
- Aureli F, & de Waal FBM (1997). Inhibition of social behavior in chimpanzees under high-density conditions. American Journal of Primatology, 41(3), 213–228. [DOI] [PubMed] [Google Scholar]
- AZA Ape TAG. (2010). Chimpanzee (Pan troglodytes) care manual Silver Spring, MD: Association of Zoos and Aquariums. [Google Scholar]
- Baker K (1996). Chimpanzees in single cages and small social groups: Effects on behavior and well-being. Contemporary Topics in Laboratory Animal Science, 35, 61–64. [Google Scholar]
- Baker KC (1997). Straw and forage material ameliorate abnormal behaviors in adult chimpanzees. Zoo Biology, 16, 225–236. [DOI] [Google Scholar]
- Baker KC (2000). Advanced age influences chimpanzee behavior in small social groups. Zoo Biology, 19(2), 111–119. [DOI] [Google Scholar]
- Baker KC (2004). Benefits of positive human interaction for socially-housed chimpanzees. Animal Welfare, 13(2), 239. [PMC free article] [PubMed] [Google Scholar]
- Baker KC, Seres M, Aureli F, & de Waal FB (2000). Injury risks among chimpanzees in three housing conditions. American Journal of Primatology, 51(3), 161–175. [DOI] [PubMed] [Google Scholar]
- Baker KC, Bloomsmith MA, Oettinger B, Neu K, Griffis C, & Schoof VAM (2014). Comparing options for pair housing rhesus macaques using behavioral welfare measures: Options for pair housing rhesus macaques. American Journal of Primatology, 76(1), 30–42. 10.1002/ajp.22190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettinger TL, Leighty KA, Daneault RB, Richards EA, & Bielitzki JT, (2017). Behavioral management: The environment and animal welfare In Schapiro SJ, (Ed.), Handbook of primate behavioral management (pp. 37–51). Boca Raton, FL: CRC Press, Taylor & Francis Group, 10.1201/9781315120652-5 [DOI] [Google Scholar]
- Bloomsmith MA, Baker KC, Leighty KA, Daneault RB, Richards EA, & Bielitzki JT, (2001). Social management of captive chimpanzees In Brent L, (Ed.), The care and management of captive chimpanzees (pp. 16–37). San Antonio, TX: American Society of Primatologists. [Google Scholar]
- Bloomsmith MA, Hasenau J, & Bohm RP (2017). Position statement: “Functionally appropriate nonhuman primate environments” as an alternative to the term “ethologically appropriate environments. Journal of the American Association for Laboratory Animal Science, 56, 102–106. [PMC free article] [PubMed] [Google Scholar]
- Boesch C, (1996). Social grouping in Tai chimpanzees In McGrew WC, Marchant LF, & Nishida T, (Eds.), Great ape societies (pp. 101–113). Cambridge, UK: Cambridge University Press, 10.1017/cbo9780511752414.010 [DOI] [Google Scholar]
- Boesch C, & Boesch-Achermann H (2000). The chimpanzees of the Taï Forest: Behavioural ecology and evolution. USA: Oxford University Press. [Google Scholar]
- Bridges JP, Mocarski EC, Reamer LA, Lambeth SP, & Schapiro SJ (2013). Weight management in captive chimpanzees (Pan troglodytes) using a modified feeding device. American Journal of Primatology, 75(Suppl 1), 51 10.1002/ajp.22188 [DOI] [Google Scholar]
- Bridges JP, Haller RL, Buchl SJ, Magden ER, Lambeth SP, & Schapiro SJ (2015). Establishing a behavioral management program for geriatric chimpanzees. American Journal of Primatology, 77(S1), 111 10.1002/ajp.22494 [DOI] [Google Scholar]
- Chapman CA, Chapman LJ, & Wrangham RW (1995). Ecological constraints on group size: An analysis of spider monkey and chimpanzee subgroups. Behavioral Ecology and Sociobiology, 36(1), 59–70. 10.1007/bf00175729 [DOI] [Google Scholar]
- Coe JC, Fulk R, & Brent L, (2001). Chimpanzee facility design In Brent L, (Ed.), The care and management of captive chimpanzees (pp. 16–37). San Antonio, TX: American Society of Primatologists. [Google Scholar]
- de Waal FB, Aureli F, & Judge PG (2000). Coping with crowding. Scientific American, 282(5), 76–81. 10.1038/scientificamerican0500-76 [DOI] [PubMed] [Google Scholar]
- Doran D (1997). Influence of seasonality on activity patterns, feeding behavior, ranging, and grouping patterns in Tai chimpanzees. International Journal of Primatology, 18(2), 183–206. 10.1023/A:1026368518431 [DOI] [Google Scholar]
- Dufour V, Sueur C, Whiten A, & Buchanan-Smith HM (2011). The impact of moving to a novel environment on social networks, activity and wellbeing in two new world primates. American Journal of Primatology, 73(8), 802–811. 10.1002/ajp.20943 [DOI] [PubMed] [Google Scholar]
- Duncan LM, Jones MA, van Lierop M, & Pillay N (2013). Chimpanzees use multiple strategies to limit aggression and stress during spatial density changes. Applied Animal Behaviour Science, 147(1–2), 159–171. 10.1016/j.applanim.2013.06.001 [DOI] [Google Scholar]
- Dye MH, (2017). Behavioral management of prosimians In Schapiro SJ, (Ed.), Handbook of primate behavioral management (pp. 435–458). Boca Raton, FL: CRC Press, Taylor & Francis Group, 10.1201/9781315120652-26 [DOI] [Google Scholar]
- Else JG (2013). A review of literature and animal welfare/regulatory requirements and guidance pertaining to the space density needs of captive research chimpanzees. Retrieved from https://dpcpsi.nih.gov/sites/default/files/ElseLitReviewFinal-110713.pdf
- Engel SR, Creson TK, Hao Y, Shen Y, Maeng S, Nekrasova T, … Chen G (2009). The extracellular signal-regulated kinase pathway contributes to the control of behavioral excitement. Molecular Psychiatry, 14(4), 448–461. 10.1038/sj.mp.4002135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson K, Gabry KE, Schulkin J, Gold P, Lindell S, Higley JD, … Suomi SJ (2005). Social withdrawal behaviors in nonhuman primates and changes in neuroendocrine and monoamine concentrations during a separation paradigm. Developmental Psychobiology, 46(4), 331–339. 10.1002/dev.20061 [DOI] [PubMed] [Google Scholar]
- Fritz J, & Howell S (1999). The behavior of captive male chimpanzees (Pan troglodytes) housed in multi-male bachelor versus mixed-sex social groups at the Primate Foundation of Arizona. American Journal of Primatology, 49(1), 54 [DOI] [Google Scholar]
- Goodall J (1986) The chimpanzees of Gombe: Patterns of behavior. Belknap Press of Harvard University Press. [Google Scholar]
- Hair JF Jr, Black WC, Babin BJ, Anderson RE, & Tatham RL (2010). Multivariate data analysis: A global perspective. New Jersey: Pearson Education. [Google Scholar]
- Honess P, (2017). Behavioral management of long-tailed macaques (Macaca fascicularis) In Schapiro SJ, (Ed.), Handbook of primate behavioral management (pp. 305–337). Boca Raton, FL: CRC Press, Taylor & Francis Group, 10.1201/9781315120652-21 [DOI] [Google Scholar]
- Hopkins WD, & Latzman RD, (2017). Future research with captive chimpanzees in the United States: Integrating scientific programs with behavioral management In Schapiro SJ, (Ed.), Handbook of primate behavioral management (pp. 139–155). Boca Raton, FL: CRC Press, Taylor & Francis Group, 10.1201/9781315120652-11 [DOI] [Google Scholar]
- Hopper LM, Freeman HD, & Ross SR (2016). Reconsidering coprophagy as an indicator of negative welfare for captive chimpanzees. Applied Animal Behaviour Science, 176, 112–119. 10.1016/j.applanim.2016.01.002 [DOI] [Google Scholar]
- Howell S, Schwandt ML, Fritz J, Roeder E, & Nelson C (2003). A stereo music system as environmental enrichment for captive chimpanzees. Lab Animal, 32(10), 31–36. 10.1038/laban1103-31 [DOI] [PubMed] [Google Scholar]
- Humle T, (2003). Behavior and ecology of chimpanzees in West Africa In Kormos R, Boesch C, Bakarr M, & Butynski TM, (Eds.), West afrrican chimpanzees (pp. 13–19). Gland, Switzerland and Cambridge, UK: International Union for Conservation of Nature and Natural Resources. [Google Scholar]
- Judge PG, & de Waal FBM (1997). Rhesus monkey behaviour under diverse population densities: Coping with long-term crowding. Animal Behaviour, 54(3), 643–662. 10.1006/anbe.1997.0469 [DOI] [PubMed] [Google Scholar]
- Lehmann J, & Boesch C (2004). To fission or to fusion: Effects of community size on wild chimpanzee (Pan troglodytes verus) social organisation. Behavioral Ecology and Sociobiology, 56(3), 10.1007/s00265-004-0781-x [DOI] [Google Scholar]
- Lukas KE, Hoff MP, & Maple TL (2003). Gorilla behavior in response to systematic alternation between zoo enclosures. Applied Animal Behaviour Science, 81(4), 367–386. 10.1016/s0168-1591(02)00237-x [DOI] [Google Scholar]
- Magden ER, Haller RL, Thiele EJ, Buchl SJ, Lambeth SP, & Schapiro SJ (2013). Acupuncture as an adjunct therapy for osteoarthritis in chimpanzees (Pan troglodytes). Journal of the American Association for Laboratory Animal Science, 52(4), 475–480. [PMC free article] [PubMed] [Google Scholar]
- Matsumoto-Oda A, Hosaka K, Huffman MA, & Kawanaka K (1998). Factors affecting party size in chimpanzees of the Mahale mountains. International Journal of Primatology, 19(6), 999–1011. 10.1023/A:1020322203166 [DOI] [Google Scholar]
- Meyer JS, Novak MA, Bowman RE, & Harlow HF (1975). Behavioral and hormonal effects of attachment object separation in surrogate-peer-reared and mother-reared infant rhesus monkeys. Developmental Psychobiology, 8(5), 425–435. 10.1002/dev.420080507 [DOI] [PubMed] [Google Scholar]
- Morgan KN, & Tromborg CT (2007). Sources of stress in captivity. Applied Animal Behaviour Science, 102(3–4), 262–302. 10.1016/j.applanim.2006.05.032 [DOI] [Google Scholar]
- Muroyama Y, & Sugiyama Y, (1994). Grooming relationships in two species of chimpanzees In Wrangham RW, McGrew WC, de Waal FBM, & Heltne PG, (Eds.), Chimpanzee cultures (pp. 169–180). Cambridge, Massachusetts and London, England: Harvard University Press in cooperation with The Chicago Academy of Sciences. [Google Scholar]
- Nakagawa S (2004). A farewell to Bonferroni: The problems of low statistical power and publication bias. Behavioral Ecology, 15(6), 1044–1045. 10.1093/beheco/arh107 [DOI] [Google Scholar]
- National Institutes of Health (2013). Council of councils working group on the use of chimpanzees in NIH-supported research: Report. https://dpcpsi.nih.gov/council/chimpanzee_research
- Neal Webb SJ, Hau J, & Schapiro SJ (2018a). Captive chimpanzee (Pan troglodytes) behavior as a function of space per animal and enclosure type. American Journal of Primatology, 80(3), e22749 10.1002/ajp.22749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal Webb SJ, Hau J, & Schapiro SJ (2018b). Refinements to captive chimpanzee (Pan troglodytes) care: A self-medication paradigm. Animal Welfare, 27(4), 327–341. 10.7120/09627286.27.4.327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuijsen K, & de Waal F (1982). Effects of spatial crowding on social behavior in a chimpanzee colony. Zoo Biology, 1, 5–28. 10.1002/zoo.1430010103 [DOI] [Google Scholar]
- Nishida T (1968). The social group of wild chimpanzees in the Mahale Mountains. Primates, 9, 167–224. 10.1007/BF01730971 [DOI] [Google Scholar]
- Nunamaker EA, Lee DR, & Lammey ML (2012). Chronic diseases in captive geriatric female chimpanzees (Pan troglodytes). Comparative Medicine, 62(2), 131–136. [PMC free article] [PubMed] [Google Scholar]
- Perneger TV (1998). What’s wrong with Bonferroni adjustments. BMJ: British Medical Journal, 316(7139)), 1236 10.1136/bmj.316.7139.1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price EE, & Stoinski TS (2007). Group size: Determinants in the wild and implications for the captive housing of wild mammals in zoos. Applied Animal Behaviour Science, 103(3–4), 255–264. 10.1016/j.applanim.2006.05.021 [DOI] [Google Scholar]
- Pruetz JDE, & McGrew WC, (2001). What does a chimpanzee need? Using natural behavior to guide the care and management of captive population In Brent L, (Ed.), The care and management of captive chimpanzees (pp. 16–37). San Antonio, TX: American Society of Primatologists. [Google Scholar]
- Reamer L, Haller R, Lambeth SP, & Schapiro SJ, (2017). Behavioral management of Pan spp In Schapiro SJ, (Ed.), Handbook of primate behavioral management (pp. 395–407). Boca Raton, FL: CRC Press, Taylor & Francis Group, 10.1201/9781315120652-24 [DOI] [Google Scholar]
- Reamer LA, Talbot CF, Hopper LM, Mareno MC, Hall K, Brosnan SF, … Schapiro SJ (2016). The effects of group size on the behavior of captive chimpanzees (Pan troglodytes). Joint meeting of the International Primatological Society and the American Society of Primatologists, August 21–27, 2016. [Google Scholar]
- Riddle KE, Keeling ME, Alford PL, & Beck TF (1982). Chimpanzee holding, rehabilitation and breeding: Facilities design and colony management. Laboratory Animal Science, 32(5), 525–533. [PubMed] [Google Scholar]
- Ross SR, Bloomsmith MA, Bettinger TL, & Wagner KE (2009). The influence of captive adolescent male chimpanzees on wounding: Management and welfare implications. Zoo Biology, 28, 623–634. 10.1002/zoo.20243 [DOI] [PubMed] [Google Scholar]
- Ross SR, Wagner KE, Schapiro SJ, & Hau J (2010). Ape behavior in two alternating environments: Comparing exhibit and short-term holding areas. American Journal of Primatology, 72(11), 951–959. 10.1002/ajp.20857 [DOI] [PubMed] [Google Scholar]
- Schapiro SJ, (2017). Introduction to the handbook of primate behavioral management In Schapiro SJ, (Ed.), Handbook of primate behavioral management (pp. 3–8). Boca Raton, FL: CRC Press, Taylor & Francis Group, 10.1201/9781315120652-2 [DOI] [Google Scholar]
- Schino G, Perretta G, Taglioni AM, Monaco V, & Troisi A (1996). Primate displacement activities as an ethopharmacological model of anxiety. Anxiety, 2(4), 186–191. [DOI] [PubMed] [Google Scholar]
- Sugiyama Y (2004). Demographic parameters and life history of chimpanzees at Bossou, Guinea. American Journal of Physical Anthropology, 124(2), 154–165. 10.1002/ajpa.10345 [DOI] [PubMed] [Google Scholar]
- Symington MM (1990). Fission-fusion social organization in Ateles and Pan. International Journal of Primatology, 11, 47–61. 10.1007/BF02193695 [DOI] [Google Scholar]
- Tutin CEG, McGrew WC, & Baldwin PJ (1983). Social organization of savanna-dwelling chimpanzees, Pan troglodytes verus, at Mt. Assirik, Senegal. Primates, 24(2), 154–173. 10.1007/BF02381079 [DOI] [Google Scholar]
- Videan EN, & Fritz J (2007). Effects of short- and long-term changes in spatial density on the social behavior of captive chimpanzees (Pan troglodytes). Applied Animal Behaviour Science, 102(1–2), 95–105. 10.1016/j.applanim.2006.03.011 [DOI] [Google Scholar]
- Watts DP, & Mitani JC (2001). Hunting behavior of chimpanzees at ngogo, kibale national park, Uganda. International Journal of Primatology, 28(1), 1–28. 10.1017/cbo9780511606397.024 [DOI] [Google Scholar]
- Williams L, & Ross CN, (2017). Behavioral management of neotropical primates: Aotus, Callithrix, and Saimiri In Schapiro SJ, (Ed.), Handbook of primate behavioral management (pp. 409–434). Boca Raton, FL: CRC Press, Taylor & Francis Group, 10.1201/9781315120652-25 [DOI] [Google Scholar]
- Wrangham RW, (1992). Living naturally: Aspects of wild environments relevant to captive chimpanzee management In Erwin J, & Landon JC, (Eds.), Chimpanzee conservation and public health: Environments for the future (pp. 71–81). Rockville, Maryland: Diagnon/Bioqual, Inc. [Google Scholar]
- Yamanashi Y, & Hayashi M (2011). Assessing the effects of cognitive experiments on the welfare of captive chimpanzees (Pan troglodytes) by direct comparison of activity budget between wild and captive chimpanzees. American Journal of Primatology, 73(12), 1231–1238. 10.1002/ajp.20995 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.