2.
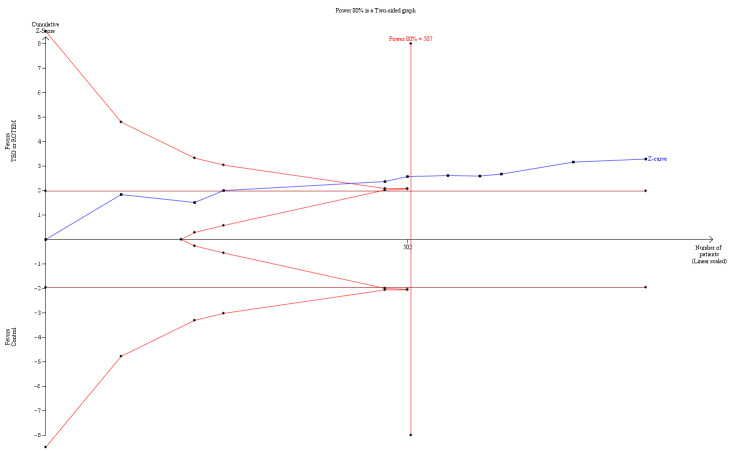
Trial Sequential Analysis (TSA) of all trials on the effect of haemostatic transfusion guided by TEG or ROTEM on the need for PRBCs resulted in a TSA alfa‐spending boundary adjusted RR of 0.86 (95% CI 0.79 to 0.95, D2= 0%, I2= 0%, fixed‐effect model) with a control event proportion of 93.3% with continuity adjustment for zero event trials (0.001 in each arm). Cumulative Z‐curve in blue crosses the monitoring boundary constructed for an adjusted information size of 507 participants corresponding to a RRR of 14% with 80% power and alpha of 0.05.