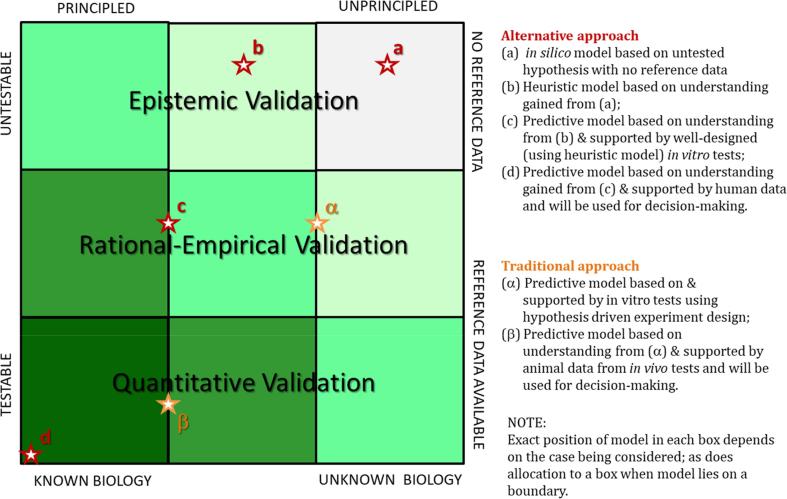
Fig. 4.
Credibility matrix showing comparative loci for a model based on traditional in vivo data-based approaches and for a model based on an alternative approach (i.e., in vitro, in silico methods, and/or micro-scale systems). The rationale for the locations of the model types, indicated by stars and letters, are given in the side-bar legend. For example, in silico models placed at the top right, might consist of a simple model ‘a’ based on a limited set of data, for instance in a QSAR. This leads to a more sophisticated, but still heuristic, model ‘b’ based on the understanding gained from model ‘a’. The predictions from models ‘a’ and ‘b’ are used to design in vitro tests that enable the development of model ‘c’, which can be validated using the rational-empirical approach, thus enhancing its credibility. Finally, this leads to the development of clinical studies and model ‘d’, supported by its predecessors and quantitatively validated or confirmed using clinical data. This places model ‘d’ in the bottom left corner, as a model whose predictions stakeholders, including regulators, practitioners, and patients, will likely use to make decisions (adapted from [42], proposed by [46]).