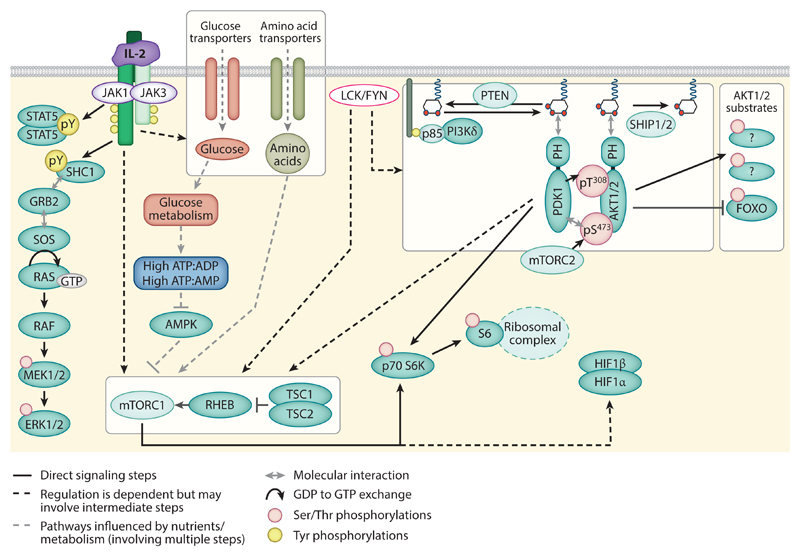
Figure 4.
Revised model for IL-2 signal transduction: IL-2 signaling outputs are likely to be modulated by other cellular signals, such as “preorganized” phosphorylation networks that do not depend on IL-2. Constitutive LCK/FYN activity has the potential to influence the phosphorylation of a number of signaling molecules in T cells and may be a key controller of the accumulation of PI(3,4,5)P3 in IL-2-dependent T cells. Levels of PI(3,4,5)P3 in cells depend on the balance in activity of phosphatidylinositol 3-kinase δ isoform (PI3Kδ or p110δ), which phosphorylates PI(4,5)P2 to generate PI(3,4,5)P3, and the PI(3,4,5)P3 phosphatases, PTEN and SHIP1/2. PI(3,4,5)P3 can coordinate the localization of a number of proteins, including the kinases phosphoinositide-dependent kinase 1 (PDK1) and AKT. Phosphorylation of T308 in the activation loop of AKT is induced by PDK1 and is coordinated by PI(3,4,5)P3 and mTORC2-mediated phosphorylation of AKT S473. Activated AKT may then go on to phosphorylate and inhibit FOXO family transcription factors, to influence transcriptional processes in T cells. However, PI3K-AKT activity in cytotoxic T lymphocytes is not a prerequisite for mTORC1 activity, and sustained activation of this complex is dependent on glucose metabolism and amino acid levels that can be regulated by IL-2 and environmental nutrient availability.