Figure 1.
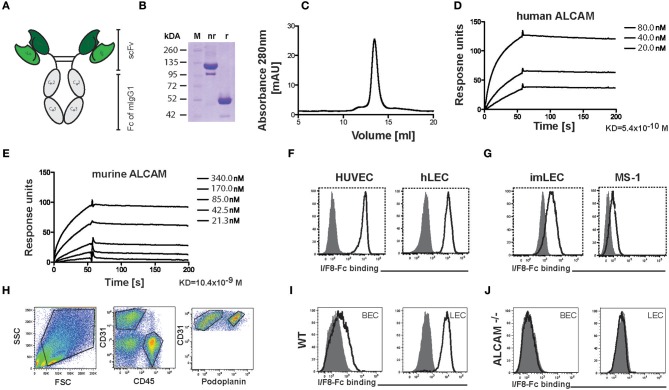
Production and characterization of the ALCAM-blocking antibody I/F8-Fc. (A) Schematic representation of the homodimeric I/F8-Fc antibody, consisting of scFv IF/8 fused to the Fc portion of murine IgG1. (B) SDS-PAGE of I/F8-Fc under non-reducing (nr) and reducing (r) conditions. Expected molecular weight: 51 kDa under reducing conditions. (C) In FPLC I/F8-Fc elutes as a homodimer. (D,E) Surface plasmon resonance profiles of purified I/F8-Fc on micro sensor chips coated with (D) human and (E) murine ALCAM. (F,G) FACS analysis reveals I/F8-Fc binding to (F) human and (G) murine endothelial cell lines. (H–J) Analysis of I/F8-Fc specificity by FACS analysis performed on single cell suspensions generated from murine lungs of WT and ALCAM−/− mice. (H) FACS gating strategy for differentiating BECs from LECs. Single-cell suspensions from enzymatically digested lung tissue were stained for CD45, CD31, and podoplanin. BECs and LECs were identified, by gating on CD45−CD31+podoplanin− (BECs) or CD45−CD31+podoplanin+ (LECs) cells. (I,J) I/F8-Fc stains BECs (CD45−CD31+podoplanin−) and LECs (CD45−CD31+podoplanin+) in lung single-cell suspension derived from (I) WT mice but not from (J) ALCAM−/− mice. Black lined histogram: I/F8-Fc, shaded histogram: KSF-Fc control. Representative data from one out of four similar experiments are shown in (F–J).