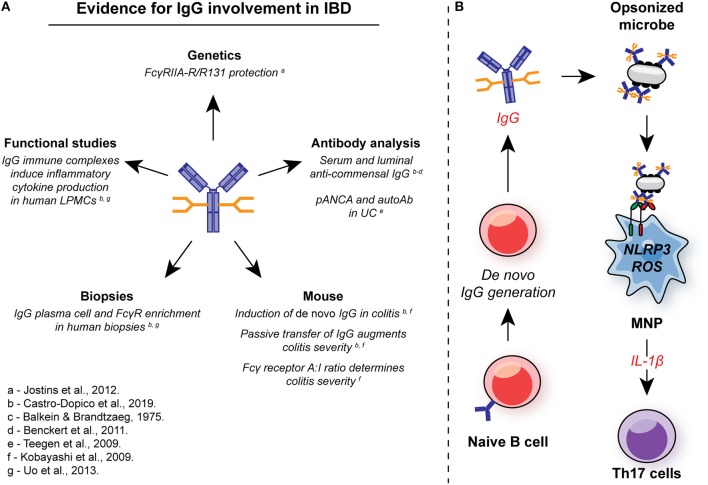
Figure 5.
Involvement of IgG in intestinal inflammation. (A) There are multiple lines of evidence for the involvement of IgG and FcγR signaling in the pathogenesis in IBD. In genetic studies, the low-affinity FcγRIIA-R131 variant is associated with protection from UC in several independent cohorts. Systemic and local induction of de novo anti-commensal IgG and auto-antibodies, such as pANCA, is observed in human IBD, while IgG+ plasma cells and FcγR-expressing cells are enriched in mucosal biopsies from IBD patients with active disease. In murine studies, de novo IgG production is observed, and passive transfer of anti-flagellin IgG to naïve animals exacerbates DSS-induced colitis. FcγR signaling strength determines the magnitude of intestinal inflammation in this model. In humans, IgG immune complex stimulation of intestinal LPMCs drives inflammatory cytokine production, including IL-1β. (B) IgG and FcγR in intestinal inflammation: Inflammation is characterized by an increase in the generation of local anti-commensal IgG. These immune complexes cross-link FcγR on colonic MNP, leading to NLRP3 and ROS-dependent IL-1β production. IL-1β, in turn drives Th17 immunity propagating inflammation. ROS, reactive oxygen species; MNP, mononuclear phagocyte; LPMCs, lamina propria mononuclear cells.