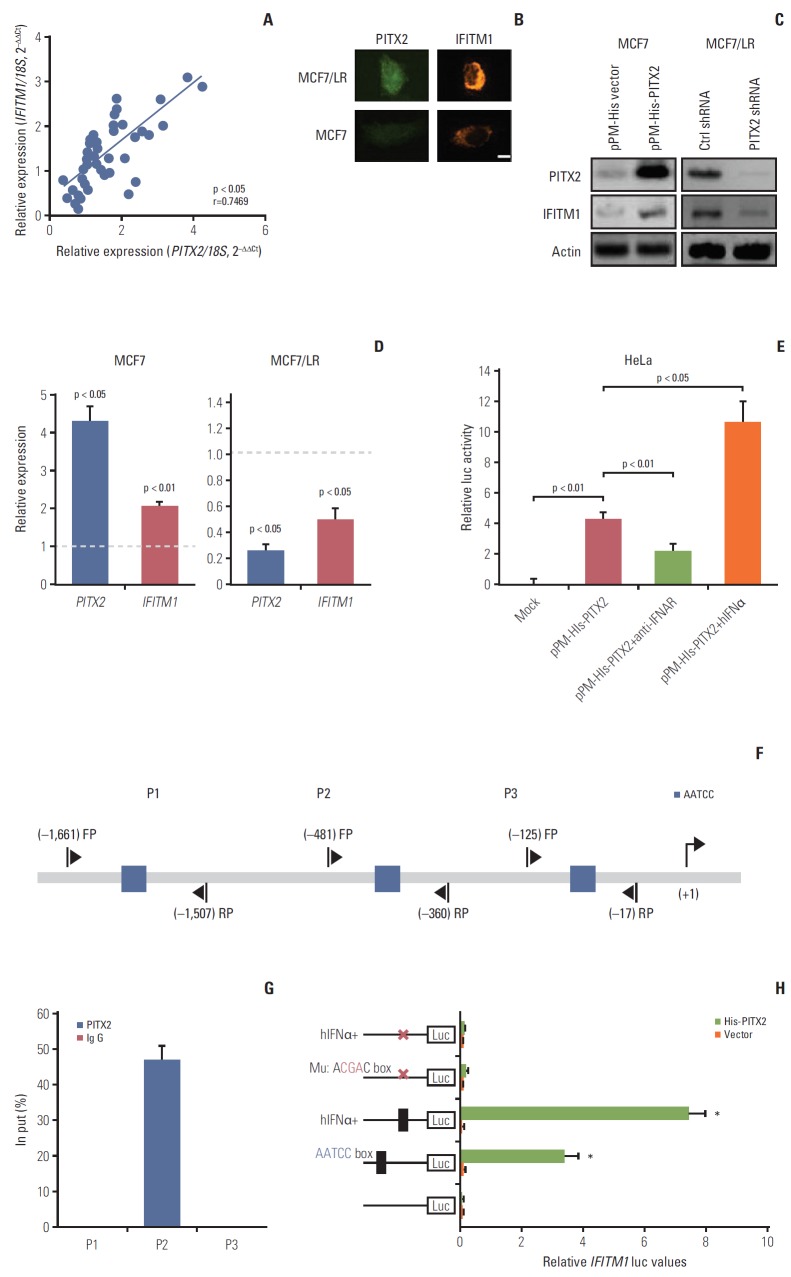
Fig. 5.
Direct regulation of interferon-inducible transmembrane protein 1 (IFITM1) transcription by paired-like homeodomain transcription factor 2 (PITX2) in breast cancer (BCa) cells. (A) PITX2 and IFITM1 expression levels in primary BCa tissues (n=24) and recurrent BCa tissues (n=20) were determined using quantitative real-time polymerase chain reaction (RT-qPCR). Fold change was determined for each sample relative to the internal control gene 18S. Each value is a mean±standard error of mean from three experiments. The correlation between PITX2 and IFITM1 expression levels were the analyzed using Pearson chi-square test. (B) Iimmunofluorescence microscopy was carried out by co-staining MCF7 and MCF7/LR cells with anti-PITX2 (green) and anti-IFITM1 (red) antibodies. Scale bar=2.5 μm. (C) Western blotting analysis of PITX2 and IFITM1 levels in MCF7/His-PITX2, MCF7/His-vector, MCF7/LR/PITX2 shRNA and MCF7/LR/scramble shRNA cells. Actin served as the loading control. (D) RT-qPCR analysis of PITX2 and IFITM1 levels in MCF7/His-PITX2, MCF7/His-vector, MCF7/LR/PITX2 shRNA, and MCF7/LR/scramble shRNA cells. Fold change was determined for each gene relative to the values in Ctrl cells (dashed line). (E) The pGL4-Luc-IFITM1 reporter plasmid and pRL-TK Renilla reporter plasmid were co-transfected with pPM-His-PITX2 or pPM-His vector into HeLa cells using FUGENE. Forty-eight hours later, cells were treated for 4 hours with 20 U/mL of hIFNα or with 5 μg/mL of anti-IFNAR neutralizing antibody, followed by measurement of luciferase activity. (F) Simplified structure of the potential binding site of PITX2 onto IFITM1 promoter. (G) MCF7/His-PITX2 cells were challenged for 4 hours with 20 U/mL of hIFNα, followed by chromatin immunoprecipitation and quantitative polymerase chain reaction analysis of recruitment of PITX2 onto IFITM1 promoter. (H) The mutation of the putative IFITM1-binding site on the promoter at –406/–402 was carried out by replacing AATCC with AcgaC using the QuikChange Site-Directed Mutagenesis Kit. The wild type or mutated pGL4-Luc-IFITM1 reporter plasmid and pRL-TK Renilla reporter plasmid were co-transfected with pPM-His-PITX2 or pPM-His vector into HeLa cells using FUGENE. Forty-eight hours later, cells were treated for 4 hours with 20 U/mL of hIFNα. Relative luciferase activity was then measured using a dual luciferase reporter assay.