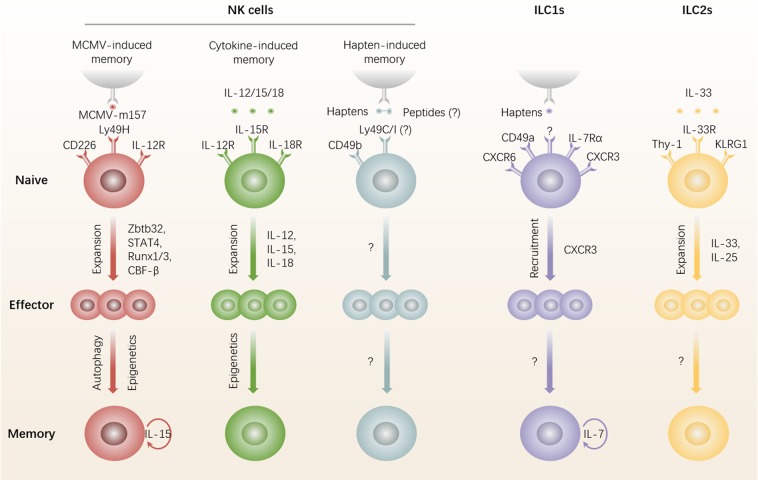
Fig. 1.
Generation and maintenance of memory ILCs. ILCs, including NK cells, ILC1s, and ILC2s, are reported to exhibit adaptive features. Ly49H+ NK cells directly recognize the MCMV-m157 protein and are completely activated with the help of costimulatory and proinflammatory cytokine signals. The transcription factors Zbtb32, STAT4, Runx1/3, and CBF-β are critical for Ly49H+ NK cell expansion. Ly49H+ NK cells subsequently undergo a contraction phase. A small population of these cells survive against apoptosis in an autophagy-dependent manner and switch to a memory state via an epigenetic program. IL-15 is required for memory Ly49H+ NK-cell longevity. Cytokines can induce non-specific memory potential in NK cells. Activation signals from combinations of cytokines induce the generation of long-lasting epigenetic imprinting of the Ifng locus. Both CD49a+ liver-resident NK cells and CD49b+ cNK cells have been reported to be responsible for hapten-induced immunological memory. CD49b+ cNK cells express Ly49C/I, which may interact with Ly49C/I-sensitive self-peptide–hapten complexes. CD49a+ liver-resident NK cells contain an IL-7Rα+ subset that conforms to the definition of ILC1s. IL-7Rα+ ILC1s, reported to highly express CXCR6 and CXCR3, acquire memory in draining LNs and maintain long-term survival in the liver. Notably, whether CD49a+ liver-resident NK cells respond locally to haptens and viruses (influenza virus, VSV, and HIV) has not been determined. Lung ST2+ ILC2s acquire memory upon IL-33 stimulation. Memory ILC2s exhibit a greater production of IL-5 and IL-13 in recall responses. cNK cells, conventional NK cells; HIV, human immunodeficiency virus type 1; ILCs, innate lymphoid cells; LN, lymph node; MCMV, mouse cytomegalovirus; NK cells, natural killer cells; VSV, vesicular stomatitis virus