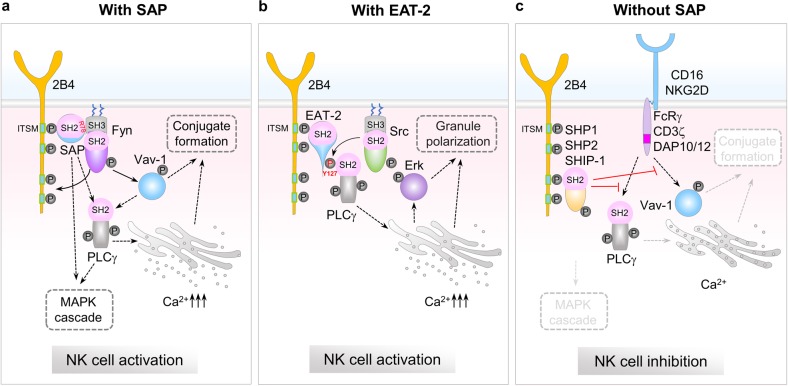
Fig. 1.
SAP-family-dependent and -independent SFR signaling in NK cell activation and inhibition. Engagement of SFRs (2B4 is shown as an example) induces tyrosine phosphorylation of cytoplasmic ITSMs, likely by Src family kinases. SH2 domain-containing proteins, SAP family adapters, or other phosphatases then bind to the phosphorylated ITSM of SFR through their SH2 domain. a SAP-dependent SFR signaling. Through arginine 78 (R78) in the SH2 domain, SAP binds to the SH3 domain of Fyn. Fyn subsequently promotes full phosphorylation of other ITSMs in SFRs, further enhancing SAP binding with Fyn. Fyn triggers Vav-1 phosphorylation and PLCγ-mediated Ca2+ flux directly or indirectly, leading to augmented NK cell conjugate formation. b EAT-2-dependent SFR signaling. EAT-2 coupling enables phosphorylation of the tyrosine in its C-terminal tail by a Src family kinase. EAT-2 associates with PLCγ through a direct interaction between phosphorylated Tyr127 in the C-terminal tail of EAT-2 and the N-terminal SH2 domain of PLCγ, which evokes calcium flux and Erk activation, leading to NK cell granule polarization. c SAP-family-independent SFR signaling. In the absence of SAP family adapters, SFRs preferentially bind to other SH2 domain-containing inhibitory molecules, such as SHP-1, SHP-2, and SHIP-1, which strongly suppress CD16- or NKG2D-mediated activating signaling