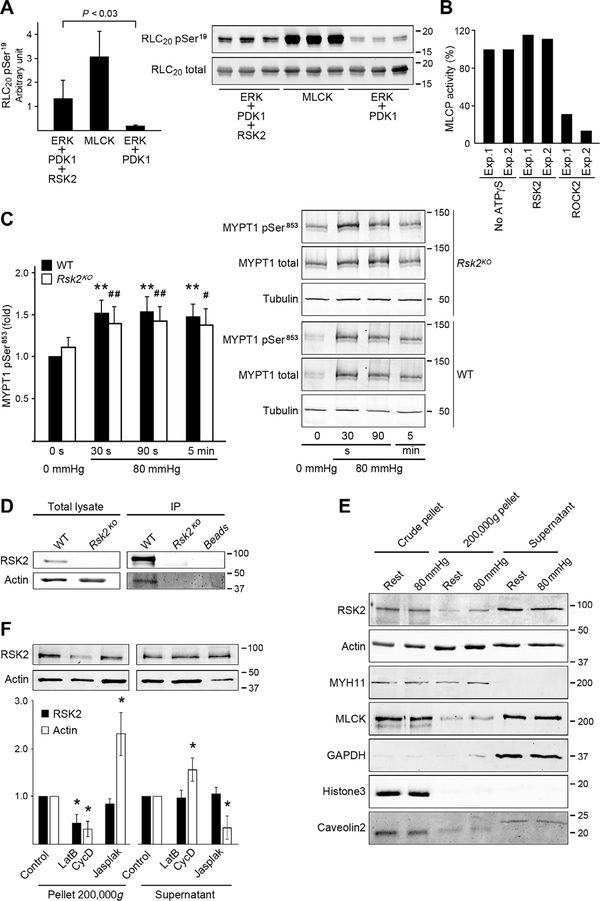
Fig. 4. RSK2 phosphorylation of RLC20, effect on MLCP activity and immunoprecipitation, and fractionation assays to assess RSK2 association with actin.
(A) Phosphorylation of purified RLC20 protein at Ser19 by recombinant active RSK2. n = 3 independent experiments each carried out in triplicate, homoscedastic Student’s t test. MLCK served as a positive control. (B) Effect of RSK2- or ROCK2-mediated phosphorylation of MYPT1 on in vitro MLCP activity. Bars show values of relative MLCP activity compared to the sample without adenosine 5′-(3-thio)triphosphate (ATPγS) thiophosphorylation. Two independent experiments each carried out in triplicate. (C) Phosphorylation of MYPT1 Thr853 (normalized to total MYPT1) over time in response to an increase in pressure from 0 to 80 mmHg in arteries from WT and Rsk2KO mice. WT: n = 8 mice; Rsk2KO: n = 5 mice. WT: **P < 0.01; Rsk2KO: #P < 0.05, ##P < 0.01, two-tailed homoscedastic Student’s t test. There was no significant difference between WT compared to Rsk2KO at any time point. (D) Representative WB of RSK2 immunoprecipitation (IP) of actin from aortic smooth muscle cell lysates from WT and Rsk2KO mice (n = 3 biological replicates). (E) WB of fractions from lysates of mesenteric arteries at 0- or 80-mmHg pressure, showing RSK2 and MLCK in the 200,000g pellet. Myosin (MYH11) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mark the cytoskeletal (200,000g pellet) and cytosolic fractions (supernatant), respectively; histone3 and caveolin2 mark the nuclear and membrane fractions and unbroken cells in the crude pellet (n = 4 mice and 4 artery arcades). (F) WB analysis of the effect of actin depolymerization by latrunculin B (LatB) plus cytochalasin D (CycD) or of actin polymerization by jasplakinolide (Jasplak) on the distribution of RSK2 and actin in the supernatant and high-speed spin pellets from mouse aortic smooth muscle cells. *P < 0.05 compared to control, two-tailed homoscedastic Student’s t test. n = 4 biological replicates. The concentrations of RSK2 in the supernatant were not significantly changed by treatment.