Abstract
Background.
Previous studies suggest that duration of antibiotic therapy for community-acquired pneumonia (CAP) often exceeds national recommendations and might represent an important opportunity to improve antibiotic stewardship nationally. Our objective was to determine the average length of antibiotic therapy (LOT) for patients treated for uncomplicated CAP in US hospitals and the proportion of patients with excessive durations.
Methods.
Records of retrospective cohorts of patients aged 18–64 years with private insurance and aged ≥65 years with Medicare hospitalized for CAP in 2012–2013 were used. Inpatient LOT was estimated as a function of length of stay. Outpatient LOT was based on prescriptions filled post discharge based on data from outpatient pharmacy files. Excessive duration was defined as outpatient LOT >3 days.
Results.
Inclusion criteria were met for 22 128 patients aged 18–64 years across 2100 hospitals and 130 746 patients aged ≥65 years across 3227 hospitals. Median total LOT was 9.5 days. LOT exceeded recommended duration for 74% of patients aged 18–64 years and 71% of patients aged ≥65 years. Patients aged 18–64 years had a median (quartile 1–quartile 3) 6 (3–7) days outpatient LOT and those aged ≥65 years had 5 (3–7) days.
Conclusions.
In this nationwide sample of patients hospitalized for CAP, median total LOT was just under 10 days, with more than 70% of patients having likely excessive treatment duration. Better adherence to recommended CAP therapy duration by improving prescribing at hospital discharge may be an important target for antibiotic stewardship programs.
Keywords: antibiotic use, community-acquired pneumonia, length of therapy, treatment duration
Community-onset infections of any kind and lower respiratory tract infections acquired in any setting are the most common indications for antibiotic use among hospitalized patients [1]. Community-acquired pneumonia (CAP), in particular, results in approximately 600 000–800 000 hospitalizations annually among adults in the United States [2, 3].
Through the Core Elements of Antibiotic Stewardship [4], the Centers for Disease Control and Prevention (CDC) advises hospitals to develop and implement recommendations for antibiotic selection and duration based on local antibiotic susceptibilities of infection-causing organisms and national guidelines. The Core Elements cite CAP as an indication for which this strategy may be particularly helpful [4].
In 2007, the Infectious Diseases Society of America (IDSA) and the American Thoracic Society (ATS) published national guidelines for treatment of CAP, recommending a minimum 5 days of therapy, with 7 or more days rarely necessary, and use of clinical stability criteria to determine when to discontinue [5]. Previous studies show that the average length of antibiotic therapy (LOT) for CAP in the United States exceeds IDSA–ATS guidelines of 5–7 days among adults hospitalized for CAP, suggesting a potentially important opportunity to improve antimicrobial stewardship. However, these studies were not generalizable to the US population [6–11]. Here, we used nationwide data to determine the LOT most commonly used for treatment of patients hospitalized for CAP in the United States.
METHODS
Study Design
We used a retrospective cohort design and administrative data to study the duration of antibiotic therapy among adult patients hospitalized for CAP and discharged in 2012 and 2013. This work was conducted under data use agreements with Truven Health Analytics and the Centers for Medicare & Medicaid Services (CMS). The work using MarketScan data was determined not to involve human patients under 45 Code of Federal Regulations (CFR) 46.102(f); therefore, institutional review board review was not required [12]. The work using CMS data was determined to be exempt from the regulations that govern the protection of human patients in research under 45 CFR 46.101(b)(5) [12].
Study Population
CAP hospitalizations were identified by selecting those with a primary diagnosis of bacterial or unspecified pneumonia (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM], diagnosis codes: 481, 482.0, 482.1, 482.2, 482.30, 482.31, 482.32, 482.39, 482.40, 482.41, 482.42, 482.49, 482.82, 482.83, 482.84, 482.89, 482.9, 483.0, 483.1, 483.8, 485, 486) [13] among patients not hospitalized in the 30 days prior to index hospitalization. We defined complicated CAP as having a complication or lack of clinical stability that required extended LOT. To operationalize this definition and restrict it to those with uncomplicated CAP, we limited it to patients with hospitalization lengths of 2–10 days, discharged home with self-care, and not rehospitalized within 3 days after the index discharge. We also excluded underlying conditions or complications that potentially warranted prolonged antibiotic courses. Specifically, we excluded patients with a cystic fibrosis diagnosis (ICD-9-CM diagnosis code 277.00, 277.01, 277.02, 277.03, 277.09), human immunodeficiency virus (HIV) diagnosis (ICD-9-CM diagnosis code 042), or an outpatient antibiotic supply that exceeded 28 days [10].
One hospitalization was selected at random for inclusion if a patient had multiple eligible hospitalizations >30 days apart during the study period. Due to data source restrictions, patients were stratified into the following 2 demographic cohorts: those aged 18–64 years with private insurance and those aged ≥65 years with Medicare.
Definition of Antibiotics
Antibacterial agents were identified in the drug files through a combination of National Drug Codes, automated text, and manual searching to account for variations in spelling of antibiotics between claims and reference data. Antibiotics were categorized into classes using the American Hospital Formulary Service Drug Information and World Health Organization drug classification systems.
Measures
The primary measures of interest were median (quartile 1–quartile 3) total LOT and proportion of patients with a LOT that exceeded 3 days of outpatient antibiotic therapy following discharge (described further in “Total length of therapy” and “Excessive Length of Therapy” sections).
Total Length of Therapy
Patient-level total LOT was calculated by summing inpatient and outpatient LOTs. Because no single data source contained both inpatient and outpatient antibiotic use information, in-patient LOT was estimated based on a function of length of stay (LOS) derived from prediction models and outpatient LOT consisted of actual days filled.
Inpatient Length of Therapy
Using linear regression, inpatient LOT was modeled as a function of LOS for both demographic cohorts using data from the MarketScan Hospital Drug Database (HDD). The HDD is a relational database developed from hospital charge detail master data that contains all charges accumulated during a hospitalization, including for pharmacy products. Patient discharge information such as diagnosis and procedure codes, patient demographics, and facility characteristics are also included. These data have been used to study inpatient pharmacy use, including probiotics [14] and antibiotics [15]. Data were limited to hospitals that submit antibiotic data directly to Truven Health, patients receiving ≥1 inpatient antibiotic, and restriction criteria listed above.
An inpatient LOT prediction table of mean LOT for each cohort was developed based on the prediction model estimates of LOT using LOS as a categorical predictor. LOS was counted as whole days using admission and discharge dates rather than days present; therefore, LOT exceeds LOS in some instances. Goodness of model fit was assessed using the R2 value. Inpatient LOT was then assigned to each patient based on the corresponding age group and LOS in the prediction table. We validated the inpatient prediction model by comparing estimated and observed inpatient LOTs among a subset of patients aged 18–64 years for whom inpatient and outpatient prescribing records could be linked for the years 2006–2013.
Outpatient Length of Therapy
To assess outpatient LOT, outpatient drug records were searched for antibiotic prescriptions filled between −1 and +3 days of discharge. If a patient had multiple outpatient antibiotic prescriptions filled during this period, the outpatient LOT was counted as the number of days with ≥1 prescription from the earliest fill date through the latest supply through date, inclusive (Supplementary Figure S1). The average proportion of total LOT contributed by the outpatient LOT was estimated by the median (quartile 1–quartile 3).
For the 18–64 year cohort, MarketScan Commercial Claims and Encounters files, drawing from hundreds of private-sector payers, were used to obtain LOS of the index hospitalization and outpatient LOT following discharge. Patients in this cohort consisted of those enrolled in private insurance with outpatient drug coverage. For the ≥65 year cohort, the 100% Medicare claims and Part D event files were used to obtain index hospitalization LOS and outpatient LOT following discharge. Patients in this ≥65 year cohort included the census of beneficiaries aged ≥65 years with traditional fee-for-service Parts A and B plus Part D Medicare coverage.
Excessive Length of Therapy
Recommended LOT is dependent on time to clinical stability [5]. Since we lacked clinical data to evaluate clinical stability directly, we used hospital discharge as a surrogate based on the assumption that patients were clinically stable at discharge [5, 16]. Our primary standard for exceeding the recommended LOT, therefore, used outpatient LOT >3 days, reasoning that LOS plus 3 days of outpatient therapy following discharge should be a patient-specific approximation of recommended LOT per IDSA–ATS guidelines [5, 9]. Number of days with excessive antibiotic use was quantified by summing patient-level outpatient LOT minus 3 days. We used a secondary standard defining recommended LOT as 7 days, since longer durations are rarely necessary according to IDSA–ATS guidelines [5].
Data Analyses
The association between categorical variables was evaluated using the χ2 test of independence. Likelihood of an event occurring was compared between 2 groups using an odds ratio. Data management and analyses were conducted using SAS, version 9.4 (SAS Institute Inc., Cary, North Carolina).
RESULTS
Length of Stay
Median (Q1–Q3) LOS was 3 (2–5) days for the 18–64 year cohort and 4 (3–5) days for the ≥65 year cohort.
Inpatient Length of Therapy
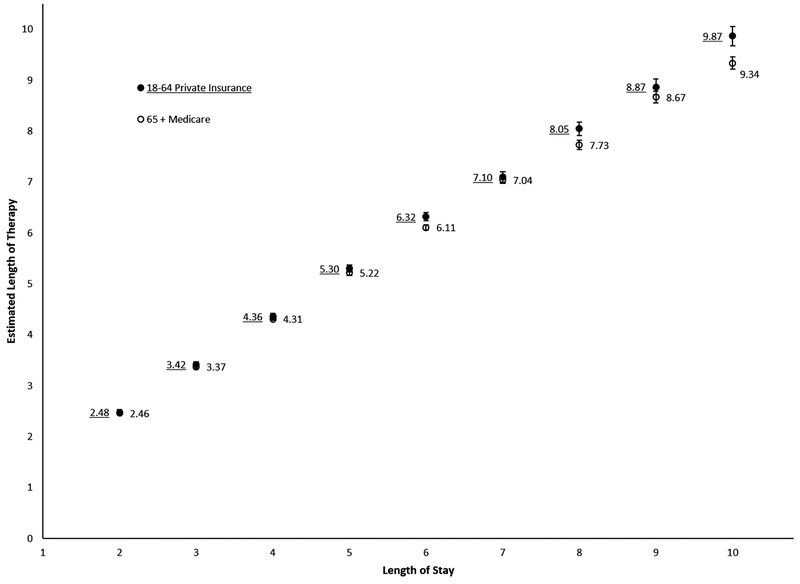
To estimate inpatient LOT, 6900 and 19 139 patient hospitalizations were included among those aged 18–64 years and ≥65 years, respectively. Predicted LOTs were generated for each value of LOS stratified by cohort (Figure 1, Supplementary Table S1). The R2 values for both models were greater than 0.7. Characteristics of the patients used in these models are shown in Supplementary Figure S2 and Supplementary Table S2. Based on these models, the estimated median (Q1–Q3) inpatient LOT was 3.4 (2.5–5.3) days for the 18–64 year cohort and 4.3 (3.4–5.2) days for the ≥65 year cohort.
Figure 1.
Prediction model of inpatient length of therapy in adults hospitalized for community-acquired pneumonia. Data were obtained from the MarketScan Hospital Drug Database. Restricted to patients receiving ≥1 inpatient antibiotic plus restriction criteria listed in the Methods section. Using linear regression, inpatient length of therapy (LOT) was modeled as a function of length of stay (LOS) as follows: LOT = β1LOS2days + β2LOS3days + β3LOS4days + β4LOS5days + β5LOS6days + β6LOS7days + β7LOS8days + β8LOS9days + β9LOS10 days. R2 values: 18–64 years, 0.78; 65+ years, 0.73.
After applying restrictions, 1177 patients aged 18–64 years had linked inpatient and outpatient prescribing records for the years 2006 through 2013 for prediction model validation. Among these, mean estimated inpatient LOT was 4.5 (95% confidence interval [CI], 4.4–4.6) days and mean actual inpatient LOT was 4.6 (95% CI, 4.4–4.7) days (Supplementary Table S3). Eighty-six percent of these patients had a predicted inpatient LOT within 1 day of the actual inpatient LOT (see Supplementary Figure S3 for agreement plot).
Outpatient Length of Therapy
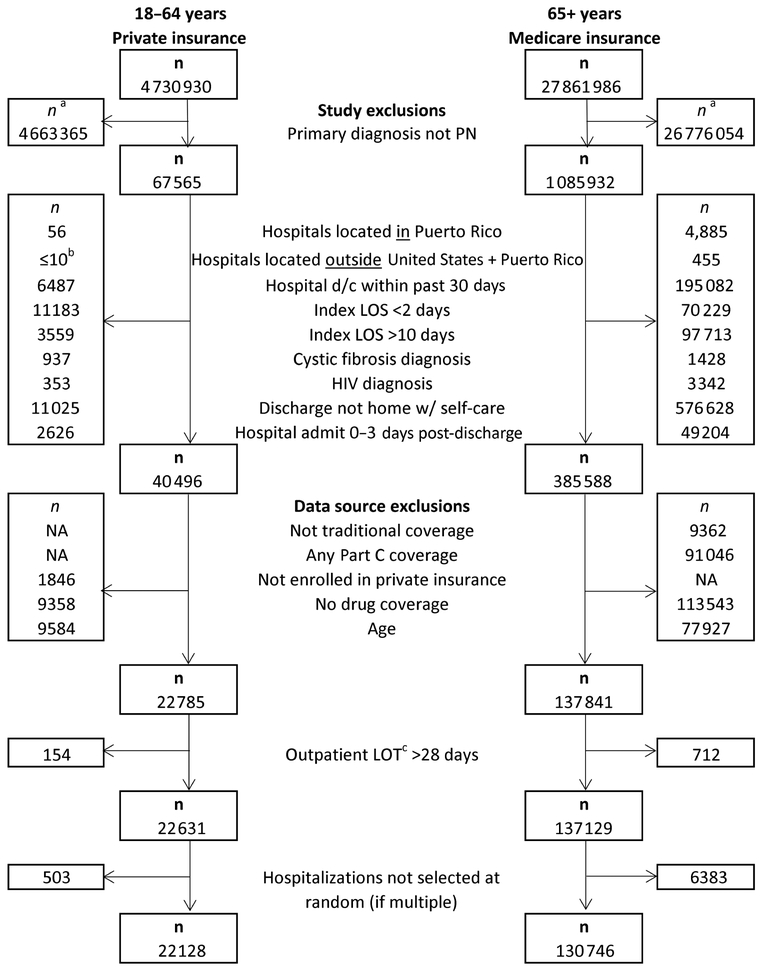
As shown in Figure 2, 22 128 and 130 746 patient hospitalizations were included to estimate outpatient and total LOT among the 18–64 year and ≥65 year cohorts, respectively. Demographic and hospitalization characteristics are listed in Table 1. Eighty percent of patients in both cohorts had at least 1 documented outpatient antibiotic fill within −1 to 3 days of discharge. The median outpatient LOT was 6 (3–7) days in the 18–64 year cohort and 5 (3–7) days in the ≥65 year cohort. Seventy-four percent of patients aged 18–64 years and 71% of patients aged ≥65 years exceeded the recommended LOT, using >3 days to define excessive, translating to 71 474 (3.2 per patient) and 375 289 (2.9 per patient) unnecessary days of antibiotic in the respective cohorts.
Figure 2.
Flow diagram of eligibility and inclusion of adults hospitalized for community-acquired pneumonia. aReasons for exclusion are not mutually exclusive. bIn accordance with the Centers for Medicare & Medicaid Services data use agreement and for consistency in presentation, actual number and corresponding percent of total were not displayed when cell sizes ≤10. cOutpatient antibiotic prescription filled within −1 to +3 days of discharge from index hospitalization. Abbreviations: d/c, discharge; HIV, human immunodeficiency virus; LOS, length of stay; LOT, length of therapy; NA, not available; PN, pneumonia.
Table 1.
Characteristics of Adults Hospitalized for Community-Acquired Pneumonia
| Category | Value | Aged 18–64 Years, Private Insurance | Aged ≥65Years, Medicare Insurance | ||
|---|---|---|---|---|---|
| (n = 22 128) | (n = 130 746) | ||||
| No. | (%) | No. | (%) | ||
| Age, y | 18–44 | 5849 | 26 | NA | |
| 45–64 | 16 279 | 74 | NA | ||
| 65–84 | NA | 104 033 | 80 | ||
| 85+ | NA | 26 713 | 20 | ||
| Sex | |||||
| Male | 10 014 | 45 | 57 945 | 44 | |
| Female | 12 114 | 55 | 72 801 | 56 | |
| Race | |||||
| White | NA | 110 330 | 84 | ||
| Black | NA | 10 406 | 8 | ||
| Other | NA | 10 010 | 8 | ||
| Healthcare use 30 d prior to hospitalization | |||||
| Outpatient claims | 8955 | 40 | 54 147 | 41 | |
| Hospice claims | 28 | 0 | 188 | 0.1 | |
| Potential healthcare-associated pneumonia | |||||
| Total | 2581 | 12 | 16 565 | 13 | |
| IV antimicrobial therapya | 1609 | 7 | 7314 | 6 | |
| IV chemotherapya | 754 | 3 | 4566 | 3 | |
| Hemodialysis | 313 | 1 | 4922 | 4 | |
| Skilled nursing facility claims | 19 | 0 | 1134 | 0.9 | |
| Index hospitalization (2–10 day LOS) | |||||
| Admission typeb | |||||
| Medical | 21 371 | 97 | 129 130 | 99 | |
| Surgical | 752 | 3 | 1615 | 1 | |
| Admission type | |||||
| Elective | NA | 8989 | 7 | ||
| Urgent | NA | 22 963 | 18 | ||
| Emergency | NA | 98 425 | 75 | ||
| Other (trauma or unknown) | NA | 369 | <1 | ||
| Admission diagnosis | |||||
| Rank 1: Pneumonia, organism unspecifiedc | NA | 67 102 | 51 | ||
| Rank 2: Shortness of breathc | NA | 18 596 | 14 | ||
| Rank 3: Coughc | NA | 6603 | 5 | ||
| Pneumonia: 481–486 | NA | 69 781 | 53 | ||
| Pneumonia, present on admission | |||||
| Present | NA | 129 415 | 99 | ||
| Not present | NA | 43 | 0.03 | ||
| Other (undetermined, insufficient info, exempt) | NA | 1288 | 1 | ||
| Intensive care unit status | |||||
| Any stay | 1396 | 6 | 25 551 | 20 | |
| No stay | 20 732 | 94 | 105 195 | 80 | |
| LOS, d | |||||
| 2 | 6466 | 29 | 30 958 | 24 | |
| 3 | 5868 | 27 | 33 762 | 26 | |
| 4 | 3848 | 17 | 24 560 | 19 | |
| 5 | 2264 | 10 | 15 815 | 12 | |
| 6 | 1441 | 7 | 10 325 | 8 | |
| 7 | 960 | 4 | 6801 | 5 | |
| 8 | 608 | 3 | 4251 | 3 | |
| 9 | 424 | 2 | 2642 | 2 | |
| 10 | 259 | 1 | 1632 | 1 | |
| Diagnosis-related group | |||||
| Median (Q1–Q3) weight | 1.0 | 1.0–1.5 | 1.0 | 1.0–1.5 | |
| 193 Simple pneumonia and pleurisy with MCC | 5191 | 23 | 30999 | 24 | |
| 194 Simple pneumonia and pleurisy with CC | 9801 | 44 | 60 917 | 47 | |
| 195 Simple pneumonia and pleurisy without CC/MCC | 5440 | 25 | 30 661 | 23 | |
| Hospitals | 2100 | 3227 | |||
| States, districtsb,d | 51 | 51 | |||
| Hospital census region | |||||
| Northeast | 3247 | 15 | 21 115 | 16 | |
| South | 9657 | 44 | 58 944 | 45 | |
| Midwest | 5726 | 26 | 30 405 | 23 | |
| West | 2608 | 12 | 20 279 | 16 | |
| Unknown | 890 | 4 | ≤10e | 0 | |
| Hospital size, number of bedsb | |||||
| 1–199 | NA | 46 758 | 36 | ||
| 200–299 | NA | 24 645 | 19 | ||
| 300–499 | NA | 33 275 | 25 | ||
| ≥500 | NA | 26 065 | 20 | ||
| Discharge diagnosesf | |||||
| Heart failure | 1668 | 8 | 30 785 | 24 | |
| Chronic obstructive pulmonary disease and bronchiectasis | 4944 | 22 | 54 236 | 41 | |
| Bronchiectasis | 313 | 1 | 3181 | 2 | |
| Empyema/lung abscess | 238 | 1 | 216 | 0.2 | |
| Asthma | 3623 | 16 | 13 886 | 11 | |
| Diabetes | 3796 | 17 | 43 327 | 33 | |
| Chronic kidney disease | 1108 | 5 | 27 311 | 21 | |
| End stage renal disease | 484 | 2 | 5371 | 4 | |
| Stroke | 248 | 1 | 5227 | 4 | |
| Liver disease, cirrhosis, and other liver conditions, viral hepatitis | 906 | 4 | 2988 | 2 | |
| Cancer/malignancy | 2571 | 12 | 32 090 | 25 | |
| Other human immunodeficiency virus/AIDS | 24 | 0 | 111 | 0 | |
| Proceduresf | |||||
| Hemodialysis | 308 | 1 | 4566 | 3 | |
| Mechanical ventilation | 111 | 0.5 | 482 | 0.4 | |
| Discharge quarter | |||||
| January-March | 6354 | 29 | 40 680 | 31 | |
| April-June | 5490 | 25 | 33 147 | 25 | |
| July-September | 4646 | 21 | 25 291 | 19 | |
| October-December | 5638 | 25 | 31 628 | 24 | |
| Follow-up (−1 to +3 days following discharge) | |||||
| Number of outpatient prescriptionsg | |||||
| 0 | 4419 | 20 | 26 390 | 20 | |
| 1 | 13 927 | 63 | 85 012 | 65 | |
| 2 | 3609 | 16 | 18 647 | 14 | |
| ≥3 | 173 | 0.8 | 697 | 0.5 | |
| Number of deaths following discharge | NA | 258 | 0.2 | ||
Abbreviations: CC, complication or comorbidity; IV, intravenous; LOS, length of stay; MCC, major complication or comorbidity; NA, not available; Q, quartile.
Includes durable medical equipment, home health, hospice, outpatient hospital, and skilled nursing services.
Missing values—Admission type: 18–64 years, n ≤ 10; ≥65 years, n ≤ 10. Hospital size: ≥65 years, n ≤ 10. Number of states: ≥65 years, n ≤ 10 patient hospitalizations.
486, pneumonia, organism unspecified; 786.05, shortness of breath; 786.2, cough.
Maximum number of states and districts is 51 due to the inclusion of the District of Columbia.
In accordance with the Centers for Medicare & Medicaid Services data use agreement and for consistency in presentation, actual number and corresponding percent of total were not displayed when cell sizes ≤10.
Constellations of diagnosis and procedure codes for each diagnosis and procedure based on the following resources:
-CCW chronic conditions: https://www.ccwdata.org/cs/groups/public/documents/document/ccw_chronic_cond_algos.pdf
-Other chronic or potentially disabling conditions: https://www.ccwdata.org/cs/groups/public/documents/document/other_cond_algos_consolidated.pdf
-Clinical classifications software for ICD-9-CM: https://www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp
Antibiotic prescriptions filled between −1 and +3 days following discharge.
Presence of outpatient antibiotic prescription was associated with LOS (P < .0001), with a longer LOS being more likely to lack an outpatient prescription (Supplementary Table S4). Of the 20% without outpatient antibiotic, only 3% obtained antibiotic through other measureable means such as outpatient intravenous therapy (Supplementary Tables S5 and S6).
Total Length of Therapy
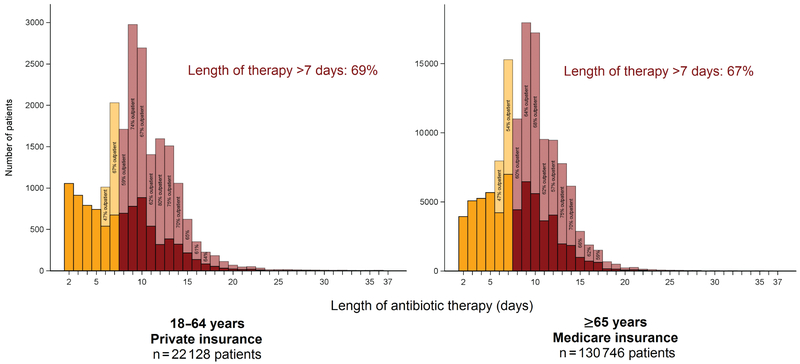
The median (Q1–Q3) total LOT, a combination of inpatient and outpatient LOT, was 9.5 (7.4–12.3) days in the 18–64 year cohort and 9.5 (7.4–11.4) days in the ≥65 year cohort (Supplementary Table S7). Sixty-nine percent of patients aged 18–64 years and 67% of patients aged ≥65 years had a LOT that exceeded 7 days (Figure 3). The outpatient LOT comprised a median 59% (41%–70%) of total LOT among those aged 18–64 years and 57% (37%–68%) among those aged ≥65 years.
Figure 3.
Total length of antibiotic therapy of adults hospitalized for community-acquired pneumonia.
Antibiotic Selection
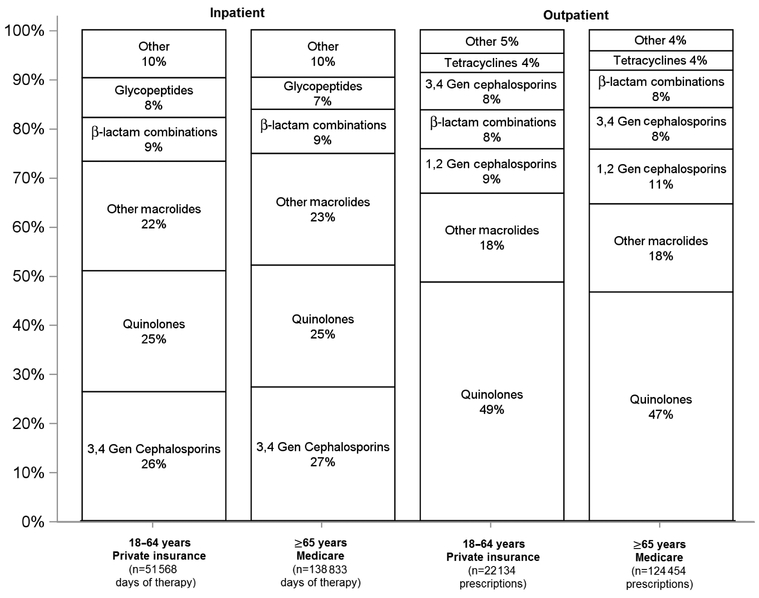
Figure 4 shows the ranking of antibiotic classes used for each setting and demographic cohort. In the inpatient setting, the top 3 antibiotic classes, accounting for 65%–75% of days of therapy, were third- and fourth-generation cephalosporins, quinolones, and macrolides for both cohorts (Supplementary Table S8). Eighty-two percent of inpatient antibiotic days of therapy were parenterally administered in both cohorts (Supplementary Figure S4).
Figure 4.
Antibiotic selection by setting and demographic among adults hospitalized for community-acquired pneumonia. Inpatient antibiotic class selection data were ranked by days of therapy. Outpatient antibiotic class selection was ranked at the prescription level. Antibiotics from separate classes that may have been used in combination therapy are treated independently. Percent selection for a given antibiotic class was calculated as follows: inpatient setting: number of days of therapy for given antibiotic class/total number of days of therapy and outpatient setting: number of prescriptions for a given antibiotic class/total number of prescriptions. β-lactam combinations refers only to β-lactam/β-lactamase combinations. Abbreviation: gen, generation.
In the outpatient setting, the top 3 antibiotic classes were quinolones, macrolides, and first- and second-generation cephalosporins for both cohorts. More than 99% of outpatient prescriptions were orally administered (Supplementary Figure S4, Supplementary Table S9).
DISCUSSION
In this study, adults hospitalized for CAP in the United States received a median of just under 10 days of antibiotic therapy in 2012–2013. More than 70% of patients exceeded the recommended duration of antibiotics according to our definition, translating to 71 474 and 375 289 unnecessary days of antibiotic for the younger and older cohorts, respectively. These findings reveal likely overtreatment with antibiotics among patients hospitalized with CAP and suggest an important target for antibiotic stewardship programs.
The CDC Core Elements for both hospital and outpatient antibiotic stewardship programs call for policies and interventions to improve antibiotic use, including appropriate duration of antibiotic therapy [4, 17]. While data on optimal LOT are lacking for many infections, the data that support effective and safe duration for antibiotic therapy are stronger for CAP. A 2007 metaanalysis of 15 inpatient and outpatient trials showed no difference in clinical failure risk between patients given antibiotic courses ≤7 days vs >7 days (relative risk, 0.89; 95% CI, 0.78–1.02) [18]. Recently, the efficacy and safety of a LOT ≤7 days was affirmed by a noninferiority randomized controlled trial of patients admitted to 4 hospitals in Spain [19]. The trial found no difference in clinical success between patients treated with an average of 5 days of antibiotics according to IDSA–ATS guidelines (day 10, 56% success; day 30, 92% success) vs patients treated based on physician discretion with an average LOT of 10 days (day 10, 49% success; day 30, 89% success). The availability of robust data on appropriate LOT for CAP, combined with CAP being a common indication for antibiotic therapy [1], make this syndrome a particularly compelling target for stewardship.
Resolution of excessively long LOTs may necessitate an approach that addresses antibiotic prescribing at discharge for patients hospitalized with CAP [20]. Based on the findings in this and prior studies, excess outpatient prescribing may be an important reason for excess LOT. As described in the Results section, 57%–59% of the total LOT consisted of outpatient therapy. In a multicenter Veteran’s Health Administration (VA) study, median outpatient, not inpatient, LOTs differed between guideline concordant and discordant groups, with median (quartile 1–quartile 3) outpatient LOTs of 3 (2–5) and 6 (4–7) days, respectively [9]. Further supporting the important role of outpatient prescribing on total LOT, other studies have shown a large portion of total duration as outpatient LOT [7] as well as decreased outpatient LOTs driving successful intervention studies [6, 11].
Approaches used in single-center antibiotic stewardship interventions have reported average LOT drops of 3 days from baseline among patients treated for CAP [6, 8, 11], which may be a feasible and effective ways to reduce LOT. These interventions include provision of education and direct feedback to medical staff and hospital attending physicians [6]; development of new guidelines with computer support promoted to hospitalists, other clinicians, and pharmacists [11]; and implementation of a patient-centered antimicrobial stewardship program [8].
Our study allows for broader generalizability than previous studies through the use of large, multifacility, nationwide cohorts. In the younger cohort, 22 128 patients across 2100 hospitals in 50 states plus the District of Columbia were represented. In the older cohort, the census of Medicare beneficiaries with traditional fee-for-service plus Part D coverage were represented, tallying to 130 746 patients across 3227 hospitals in 50 states plus the District of Columbia. Previous studies have been limited to predominantly male patients admitted to VA hospitals [8, 9], single hospitals [6–8], or international studies not separately reporting the findings of US hospitals [10]. Despite differences in study design, the findings of this study corroborate with those of the smaller, less representative studies. The largest of the US studies—a retrospective study of 30 US VA hospitals that included more detailed clinical information—had a median LOT of 10 (8–12) days, with 93% of patients exceeding LOT guidelines [9]. Other studies reported median LOTs of 10 days [6, 7] and mean LOTs of 11–12 days [8, 10].
While reliance on administrative data allowed for nationwide estimates, which is a strength of this study, limitations associated with administrative data should be acknowledged. We identified potential CAP patients using a constellation of ICD-9-CM codes. These codes have good sensitivity (84%) and positive predictive value (92%) for pneumonia when listed as the first diagnosis code [21]. However, there is potential for misclassification since we were unable to confirm the diagnosis using clinical data (eg, imaging results, symptoms, and white blood cell count). Furthermore, we may not have captured all discharge antibiotic prescription fills since some may have been obtained without using the outpatient prescription drug benefit, thus the median intended LOT could be underestimated. Also due to data limitations, we were unable to evaluate LOT among patients without health insurance or with health insurance but without drug coverage.
Although clinical data were not available to define clinical stability, we used discharge, a point at which clinical stability should be met, to approximate clinical stability. Prior studies have shown the median time to clinical stability in CAP patients to be 3 days, with most patients reaching clinical stability prior to discharge [16, 22], suggesting our surrogate for clinical stability may be conservative.
We were limited in our ability to exclude healthcare-associated and hospital-acquired pneumonia (HCAP and HAP) since the relevant information was limited or not available across all data sources. Patients classified with HCAP would include those who resided in a nursing home or long-term care facility or those who received hemodialysis, intravenous (IV) antibiotics, IV chemo-therapy, or wound care in the 30 days [23] before index admission. Patients with HAP due to the index hospitalization would include those newly infected at least 48 hours after hospital admission [23]. Using available data (as shown in Table 1), approximately 12%–13% of patients may have met 1 or more criteria for HCAP. Median LOT did not differ between patients with and without potential HCAP for both age cohorts (data not shown). Only 0.03% of the ≥65 year cohort could be classified as a HAP due to having a primary diagnosis of pneumonia that was not present on admission. Based on these numbers, it is likely that only a small portion of the patients included in this study were misclassified as CAP.
We attempted to exclude patients who may appropriately require prolonged antibiotic therapy, such as patients with cystic fibrosis, symptomatic HIV, or more complicated cases as suggested by prolonged hospitalizations or readmissions. We did not, however, exclude all risk factors for complicated CAP that might merit longer treatment durations, such as other immune deficiencies, structural lung disease/bronchiectasis, empyema/lung abscess, and chronic steroid use. Nevertheless, as shown in Table 1, other HIV/AIDS, bronchiectasis, empyema, and lung abscesses were rare across the cohorts and therefore should not account for the overall long treatment durations.
The majority of adult patients treated for CAP in US hospitals in 2012–2013 had antibiotic therapy durations that exceeded recommendations. These findings suggest prolonged and potentially excessive antibiotic treatment among patients hospitalized with uncomplicated CAP. Stewardship at the time of discharge may be an important target for antibiotic stewardship programs. Optimization of the duration of therapy, combined with optimal antibiotic selection and dosing, should help to maximize benefits to the patient while minimizing potential harms.
Supplementary Material
Acknowledgments
Financial support. This work was supported through salary funds from the Division of Healthcare Quality Promotion of the CDC.
Footnotes
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Publisher's Disclaimer: Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Magill SS, Edwards JR, Beldavs ZG, et al. Prevalence of antimicrobial use in US acute care hospitals, May–September 2011. JAMA 2014; 312:1438–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jain S, Self WH, Wunderink RG, et al. ; CDC EPIC Study Team. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med 2015; 373:415–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agency for Healthcare Research and Quality. Weighted national estimates from Healthcare Cost and Utilization Project National Inpatient Sample, 2013. Available at: http://hcupnet.ahrq.gov. Accessed 7 October 2016.
- 4.Centers for Disease Control and Prevention. Core Elements of Hospital Antibiotic Stewardship Programs. Available at: http://www.cdc.gov/getsmart/healthcare/implementation/core-elements.html. Accessed 23 October 2016.
- 5.Mandell LA, Wunderink RG, Anzueto A, et al. ; Infectious Diseases Society of America; American Thoracic Society. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007; 44(Suppl 2):S27–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Avdic E, Cushinotto LA, Hughes AH, et al. Impact of an antimicrobial steward-ship intervention on shortening the duration of therapy for community-acquired pneumonia. Clin Infect Dis 2012; 54:1581–7. [DOI] [PubMed] [Google Scholar]
- 7.Jenkins TC, Stella SA, Cervantes L, et al. Targets for antibiotic and healthcare resource stewardship in inpatient community-acquired pneumonia: a comparison of management practices with National Guideline Recommendations. Infection 2013; 41:135–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kurtzhalts KE, Sellick JA Jr, Ruh CA, Carbo JF, Ott MC, Mergenhagen KA. Impact of antimicrobial stewardship on outcomes in hospitalized veterans with pneumonia. Clin Ther 2016; 38:1750–8. [DOI] [PubMed] [Google Scholar]
- 9.Madaras-Kelly KJ, Burk M, Caplinger C, et al. ; Pneumonia Duration of Therapy Medication Utilization Evaluation Group. Total duration of antimicrobial therapy in veterans hospitalized with uncomplicated pneumonia: results of a national medication utilization evaluation. J Hosp Med 2016; 11:832–9. [DOI] [PubMed] [Google Scholar]
- 10.Aliberti S, Blasi F, Zanaboni AM, et al. Duration of antibiotic therapy in hospitalised patients with community-acquired pneumonia. Eur Respir J 2010; 36:128–34. [DOI] [PubMed] [Google Scholar]
- 11.Haas MK, Dalton K, Knepper BC, et al. Effects of a syndrome-specific antibiotic stewardship intervention for inpatient community-acquired pneumonia. Open Forum Infect Dis 2016; 3: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Office for Human Research Protections. 45 CFR part 46 Protection of Human Subjects. Available at: http://www.hhs.gov/ohrp/policy/ohrpregulations.pdf. Accessed 30 December 2015.
- 13.Centers for Medicare & Medicaid Services, the Joint Commission. The Specifications Manual for National Hospital Inpatient Quality Measures [Version 4.4a, January, 2015]. Available at: https://www.qualitynet.org/dcs/ContentServer?c=Page&pagename=QnetPublic%2FPage%2FQnetTier3&cid=1228773989482. Accessed 19 February 2016.
- 14.Yi SH, Jernigan JA, McDonald LC. Prevalence of probiotic use among inpatients: a descriptive study of 145 U.S. hospitals. Am J Infect Control 2016; 44:548–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baggs J, Fridkin SK, Pollack LA, Srinivasan A, Jernigan JA. Estimating national trends in inpatient antibiotic use among US hospitals from 2006 to 2012. JAMA Intern Med 2016; 176:1639–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aliberti S, Zanaboni AM, Wiemken T, et al. Criteria for clinical stability in hospitalised patients with community-acquired pneumonia. Eur Respir J 2013; 42:742–9. [DOI] [PubMed] [Google Scholar]
- 17.Sanchez GV, Fleming-Dutra KE, Roberts RM, Hicks LA. Core elements of outpatient antibiotic stewardship. MMWR Recomm Rep 2016; 65(6):1–12. [DOI] [PubMed] [Google Scholar]
- 18.Li JZ, Winston LG, Moore DH, Bent S. Efficacy of short-course antibiotic regimens for community-acquired pneumonia: a meta-analysis. Am J Med 2007; 120:783–90. [DOI] [PubMed] [Google Scholar]
- 19.Uranga A, España PP, Bilbao A, et al. Duration of antibiotic treatment in community-acquired pneumonia: a multicenter randomized clinical trial. JAMA Intern Med 2016; 176:1257–65. [DOI] [PubMed] [Google Scholar]
- 20.Slayton RB, Toth D, Lee BY, et al. Vital signs: estimated effects of a coordinated approach for action to reduce antibiotic-resistant infections in health care facilities—United States. Morb Mortal Wkly Rep 2015; 64:826–31. [PMC free article] [PubMed] [Google Scholar]
- 21.Whittle J, Fine MJ, Joyce DZ, et al. Community-acquired pneumonia: can it be defined with claims data? Am J Med Qual 1997; 12:187–93. [DOI] [PubMed] [Google Scholar]
- 22.Halm EA, Fine MJ, Marrie TJ, et al. Time to clinical stability in patients hospitalized with community-acquired pneumonia: implications for practice guidelines. JAMA 1998; 279:1452–7. [DOI] [PubMed] [Google Scholar]
- 23.American Thoracic Society and Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 2005; 171:388–416. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.