1. Introduction and Background
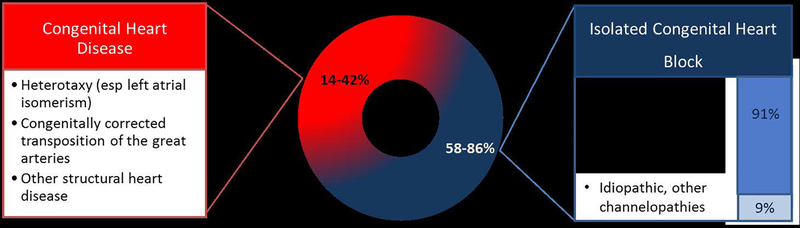
Congenital complete heart block (CCHB) is a rare cardiac disorder affecting 1 in 15–20,000 patients (Michaëlsson & Engle, 1972). It is one of the most dangerous fetal bradyarrhythmias due to the increased risk of fetal hydrops and fetal loss (Brucato, Cimaz, Caporali, Ramoni, & Buyon, 2011; Eliasson et al., 2011). CCHB implies third degree heart block, which involves complete atrioventricular (AV) dissociation secondary to disruption of the AV node and results in significant fetal bradycardia with heart rates of generally 40–90 beats per minute (bpm), depending on the ventricular escape rhythm (Brito-Zerón, Izmirly, Ramos-Casals, Buyon, & Khamashta, 2015; Buyon et al., 1998). Maternal risk factors for the development of CCHB include metabolic disease (i.e. type 2 diabetes mellitus), medication exposures (i.e. anti-convulsants and retinoic acids), viral infections, and autoantibody transfer. CCHB is also associated with structural heart abnormalities including diagnoses such as congenitally corrected transposition of the great arteries (CCTGA), complete AV canal defects, and left atrial isomerism (heterotaxy syndrome) in ~14–42% of cases (Brucato et al., 2003). Channelopathies represent a rare cause of CCHB, which can involve genetic variants of ion channel genes including mutations in SCN5A, SCN1B, SCN10A, TRPM4, KCNK17, and the genes encoding connexin proteins (Baruteau, Probst, & Abriel, 2015; Makita et al., 2012; Schott et al., 1999) (Baruteau, Behaghel, et al., 2012; Baruteau, Fouchard, et al., 2012). Overall, the most common cause of isolated CCHB block is fetal exposure to autoimmune maternal antibodies, which accounts for up to 91% of isolated CHB (Brucato et al., 2011; Buyon et al., 1998). (Figure 1)
Figure 1:
Congenital complete heart block is associated with congenital heart disease in ~14–42% of cases (Brucato et al., 2003). The rest are isolated congenital heart block. 91% of isolated congenital heart block cases are associated with autoimmune mediated fetal heart block (Brucato et al., 2011; Buyon et al., 1998).
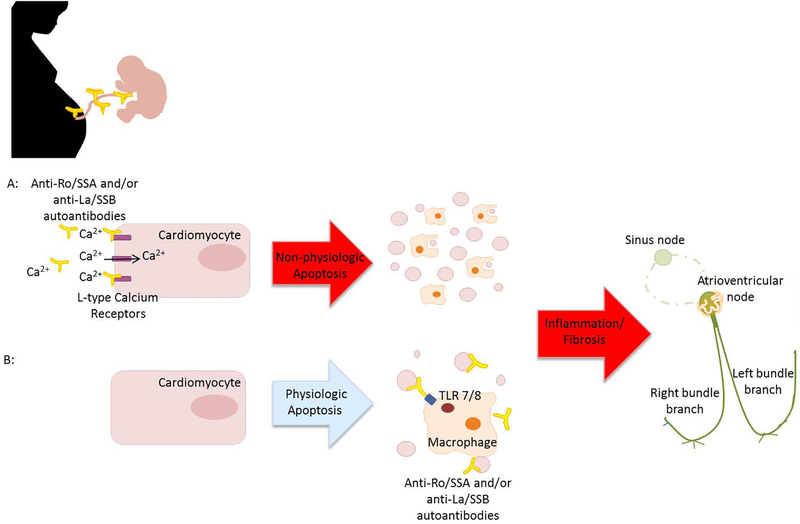
Immune mediated CCHB is caused by passive placental transfer of maternal autoantibodies specific for Ro (Sjögren-syndrome-related antigen A, SSA) and LA antigens (Sjögren-syndrome-related antigen B, SSB) and can occur as early as 11 weeks gestation. It is hypothesized that the autoantibodies bind to L-type calcium channels in the fetal conduction tissue, thereby increasing the internalization of calcium channels, which in turn disturbs cytoplasmic calcium metabolism. This leads to non-physiologic apoptosis and results in inflammation (Baruteau et al., 2016). An alternative hypothesis suggests fetal SSA/Ro and SSB/La ribonucleoproteins are brought to the cell surface during physiologic cell apoptosis and opsonized by maternal anti-SSA/Ro and anti-SSB/LA antibodies then phagocytosed by fetal macrophages. The ssRNA associated with these ribonucleoproteins activates the toll-like receptor (TLR) 7/8 pathway triggering secretion of proinflammatory and profibrotic cytokines resulting in inflammation (Alvarez et al., 2011; Clancy et al., 2010; Izmirly et al., 2017). Both of these hypotheses result in a common pathway of inflammation leading to fibrosis and scarring of the fetal conduction system, which can result in CCHB. (Figure 2) Fetal heart block usually develops between 16 and 24 weeks of gestation although later onset can occur (Rein et al., 2009) . Only 2–5% of fetuses will develop CHB in autoantibody positive mothers. The risk increases to 12–15% in fetuses of mothers with previous children with CCHB. In general, only a third of mothers of CCHB fetuses have an identified autoimmune disorder such as Sjogren’s or Lupus at the time of CCHB diagnosis in their fetus (Buyon et al., 1998).
Figure 2:
Maternal Anti-Ro/SSA and/or anti-La/SSB autoantibodies pass through the placenta . It is hypothesized that they A: bind to L-type Calcium Receptors on fetal cardiomyocytes preventing uptake of calcium possibly leading to cellular apoptosis. Alternatively, B: they may bind to internal target antigens released during physiologic apoptosis which are then opsonized by maternal circulating antibodies. This leads to activation of the TLR 7/8 cascade. Both hypotheses result in inflammation, fibrosis and eventual calcification of the cardiac system resulting in CHB.
Autoimmune CCHB has a reported cumulative mortality of around 19% of which approximately 70% die in utero according to aggregate data taken from a systematic review of the literature (Brito-Zerón et al., 2015). Mortality is increased when there is antibody-associated myocardial inflammation in addition to CCHB (Brito-Zerón et al., 2015; Buyon et al., 1998; Jaeggi, Hamilton, Silverman, Zamora, & Hornberger, 2002; Schmidt, Ulmer, Silverman, Kleinman, & Copel, 1991). Specific risk factors associated with in utero mortality include fetal hydrops, diagnosis of CHB at <20 weeks, ventricular escape rate <55 bpm, and impaired left ventricular function (Eliasson et al., 2011). Atrioventricular valvar tensor apparatus dysfunction is a severe complication seen in 1.6% of patients with autoimmune CCHB and leads to significant valve regurgitation (Cuneo et al., 2011). The US Research Registry for Neonatal Lupus found that cases of CCHB had a mortality rate >50% when the fetus had either dilated cardiomyopathy or endocardial elastofibriosis (EFE) and a mortality of 100% when the fetus had both risk factors (Izmirly et al., 2011). Postnatally these patients require close monitoring as their mortality risks continue after birth. Almost two thirds of these patients will require a pacemaker, the majority within the first 10 days of life (Brito-Zerón et al., 2015; Eliasson et al., 2011). However, with pacemaker implantation, most of these children have an overall good prognosis and can be expected to have a near normal life expectancy if ventricular function is preserved (D. Friedman, Duncanson, Glickstein, & Buyon, 2003).
2. Prenatal evaluation of complete heart block
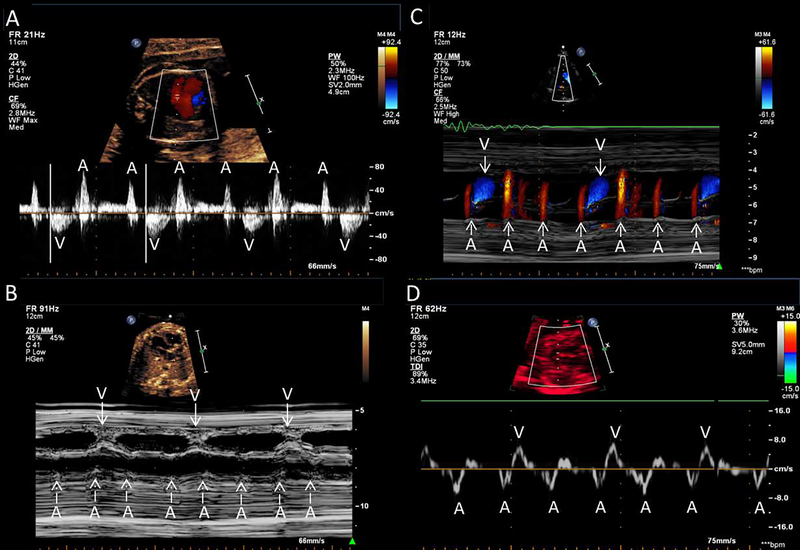
Fetal echocardiography is the standard tool for diagnosis and management of fetal bradyarrhythmias including CCHB. Fetal echocardiography evaluates the mechanical consequences of the arrhythmia rather than directly detecting the conduction itself. This is achieved through M-mode and/or Doppler echocardiography. In M-Mode, the atrial (a-wave) and ventricular (v-wave) wall motion during contraction are measured simultaneously and displayed along a continuous time scale in order to identify the ratio of a- to v-waves, thus allowing for measurement of time intervals (a–v time, v–a time). Fetal movement and position can make accurate M-mode cursor alignment difficult, and M-mode is also limited in its ability to evaluate the onset and peak of atrial and ventricular contractions. Pulsed wave Doppler echocardiography measures simultaneous flow across the mitral and aortic valve and, in the case of CCHB, demonstrates dissociation of atrial inflow and ventricular outflow. This can also be done with simultaneous Doppler flows in the superior vena cava and aorta as well as in the pulmonary vein and pulmonary artery. For patients with lower degrees of heart block (including first degree and second degree), measurements of the difference between the onset of the atrial and ventricular waveforms are used to calculate PR intervals. Color Doppler M-mode incorporates color flow Doppler superimposed on the 2D wall motion across a continuous time scale which is another helpful tool in the evaluation of CCHB (Fyfe, Meyer, & Case, 1993), (Dancea, Fouron, Miró, Skoll, & Lessard, 2000; Strasburger & Wakai, 2010; Wacker-Gussmann, Strasburger, Cuneo, & Wakai, 2014). Tissue Doppler imaging can directly record the mechanical activity of the atria and ventricles during the cardiac cycle allowing for more accurate measurement of cardiac intervals and localization of arrhythmias (Rein et al., 2002). (Figure 3)
Figure 3:
Prenatal evaluation for CCHB A: Pulsed Wave Doppler. B: M- mode C: Color Doppler M-mode. D: Tissue Doppler Imaging. A, atrial; V, ventricular.
Diagnosing progression to CCHB in at-risk pregnancies is challenging due to its sudden and unpredictable progression from normal sinus rhythm to first degree heart block and then further to CCHB. It is due to this rapid conversion to CCHB that serial fetal echocardiography has been shown to be an imperfect tool even with weekly screening (Cuneo et al., 2010). Fetal electrocardiography (fECG) via direct placement of electrodes on the maternal abdomen can be used to directly assess fetal conduction as early as 17 weeks, but is not a tool used commonly in clinical practice. This technique is limited by fetal movement leading to loss of signal as well as difficulty in distinguishing fetal from maternal ECG signals. A fECG can also be performed by placing a scalp lead on patients with GA >36wks (Budin & Abboud, 1994) which is helpful in peripartum CCHB management, but not useful for screening or monitoring CCHB earlier in gestation. Another technique called fetal magnetocardiography records the magnetic field produced by the fetal heart to produce high quality ECG like waveforms. This technology is restricted by the requirement of expensive and specialized equipment only available at a select few locations (Donofrio et al., 2014; Hornberger & Collins, 2008; Zhao et al., 2008).
Another promising approach to diagnosing CCHB is institution of a home monitoring program in which the mother is trained to measure fetal heart rate twice a day using a simple handheld Doppler system. These studies have been shown to be feasible with up to 87% of patients completing the monitoring protocol (Cuneo et al., 2010; Cuneo, Moon-Grady, et al., 2017; Cuneo et al., 2018). In one study, this was used to successfully identify progression to CCHB in the at-risk fetus allowing for emergent steroid treatment and reversion to sinus rhythm in one of the patients (Cuneo, Ambrose, & Tworetzky, 2016; Cuneo et al., 2018). A wearable, transabdominal wireless fetal monitor has been developed which is able to differentiate maternal and fetal heart rates allowing for continuous fetal ECG monitoring for gestational ages greater than 20 weeks with decreased accuracy after 26 weeks (Graatsma, Jacod, van Egmond, Mulder, & Visser, 2009). The monitor was able to accurately identify fetal bradycardia in seven of nine tracings in patients with CHB and was also able to identify p waves in addition to QRS complexes in one of the patients (Narayan, Vignola, Fifer, & Williams, 2015). Further work is needed to confirm the usefulness and accuracy of this device in diagnosing fetal arrhythmias.
3. Fetal Pharmacotherapy for CHB
Despite advances in technology allowing for earlier and more accurate identification of CCHB, there remains no standardized medical therapy for treating this disease. The goal of fetal therapy is multi-fold: 1) prevent progression to higher degrees of AV block; 2) decrease risk for clinical deterioration and progression to hydrops resulting in reduced morbidity and mortality. The mainstay of pharmacological management in CCHB is corticosteroids, specifically fluorinated glucocorticoids such as dexamethasone or betamethasone, which are anti-inflammatory drugs with the ability to cross the placenta. Corticosteroids may reduce immune-mediated damage to the AV conduction tissue and myocardial tissue that leads to myocarditis and cardiomyopathy in CCHB. Corticosteroids are often initiated when a fetus develops first or second degree block in attempts to prevent progression to CCHB, though there is limited evidence for regression or resolution of heart block in these populations (Ciardulli et al., 2018; Eliasson et al., 2011). Corticosteroid administration can have negative side effects on the mother and fetus, such as increased risks of maternal diabetes, maternal hypertension and hyperaldosteronism. Additionally, there is evidence that exposure to in utero corticosteroids can impair fetal neurological development (Costedoat-Chalumeau et al., 2003; Modi et al., 2001; Whitelaw & Thoresen, 2000) .
Once a fetus has developed CCHB, it is generally found to be irreversible. Thus, pharmacological therapy for CCHB is aimed at limiting further immune mediated damage through the use of corticosteroids, and at times augmenting cardiac output via beta stimulation of heart rate. Two contemporary retrospective cohort studies evaluated the treatment of patients with congenital heart block with dexamethasone plus beta stimulation for HR <55bpm versus no treatment. One study found this treatment strategy improved 1 year survival and showed that half of beta stimulated fetuses had a small increase in heart rate during treatment (Jaeggi et al., 2004). Conversely, the other study found no difference in outcomes(Rosenthal, Gordon, Simpson, & Sharland, 2005). Both studies were limited by small size and lack of standardized treatment protocols. Another retrospective study used similar pharmacologic treatment strategy of corticosteroids plus beta-agonist stimulation for HR <56bpm and added digoxin +/− IVIG treatment for heart failure progression in fetuses of SSA/SSB antibodies. They also instituted strict fetal monitoring guidelines including weekly echocardiograms and defined early delivery indicators including progression of fetal hydrops. This cohort had significant improvement in mortality in comparison to historical controls, potentially due to close monitoring of the fetuses and delivery for signs of fetal compromise (Cuneo et al., 2010). A large multinational, multicenter retrospective study of patients with second degree heart block or CCHB found no difference in mortality between those treated with corticosteroids +/− beta stimulation versus untreated populations (Eliasson et al., 2011). Additionally, a recent meta-analysis comparing data from 8 studies for a total of 162 fetuses found no difference in mortality or pacemaker implantation in steroid-treated versus non-treated patients (Ciardulli et al., 2017). Despite these generally negative findings, corticosteroid +/− beta stimulation may prove to be advantageous in certain CCHB subsets such as those with fetal hydrops (Ciardulli et al., 2017) or those treated during emergent CCHB (Cuneo et al., 2016). Thus, ongoing investigation is important to identify CCHB patients who may possibly benefit from optimal pharmacotherapy.
Alternative therapeutic strategies to fetal corticosteroids such as plasmapheresis and intravenous gamma globulin (IVIG) are aimed at reducing circulating levels of maternal autoimmune antibodies and decreasing placental transmission. These treatments may also accelerate maternal IgG catabolism (Tran, Cavill, Buyon, & Gordon, 2004). A case-control study of anti SSA/SSB antibody positive pregnant women treated with fluorinated corticosteroids and/or plasmapheresis started prior to pregnancy suggested treatment may help decrease the risk of developing CHB (Makino, Yonemoto, Itoh, & Takeda, 2007). In a multicenter open label study of preventative IVIG Therapy for Congenital Heart Block (PITCH study), pregnant women with anti-SSA or anti-SSB antibodies and previous pregnancy with fetal CHB or neonatal lupus rash were treated with IVIG every 3 weeks. However, the study was halted early as IVIG was not effective in preventing CHB (D. M. Friedman et al., 2010). A similar study performed in London also showed no difference in the development of CHB in patients treated with IVIG compared to controls (Pisoni et al., 2010). Conversely, a prospective study of anti SSA/SSB antibody positive pregnant females treated with a protocol of weekly plasmapheresis, biweekly IVIG, and daily betamethasone with IVIG also administered after delivery found half of the patients with second degree heart block reverted to first degree heart block or normal sinus rhythm (Ruffatti et al., 2016; Ruffatti et al., 2012). Both IVIG and plasmapheresis have been demonstrated to be safe in pregnant women with autoimmune diseases, but are expensive, difficult to administer, and can lead to hemodynamic compromise as well as risks of immunosuppression (El-Haieg, Zanati, & El-Foual, 2007; Gürcan, Keskin, & Ahmed, 2010; Perricone et al., 2008). The variable results and lack of reproducibility of these studies, potentially in part secondary to low numbers of subjects, do not provide strong enough evidence for use as primary therapy for preventing CCHB at this time.
Another approach to decreasing the risk for developing fetal CHB in pregnancies complicated by autoimmune antibodies is through the use of hydroxychloroquine (HCQ). This antimalarial agent used to treat systemic lupus erythematous inhibits several TLR pathways suggested to be important in the macrophage induced fibrosis and inflammation seen in immune mediated CCHB (Lafyatis, York, & Marshak-Rothstein, 2006). A recent case-control study suggested it may decrease the risk of CHB (Izmirly et al., 2010). A multinational historical cohort study further showed a 70% reduction in CHB recurrence in pregnant patients with a previous child with CHB treated with HCQ. No fetal deaths were seen in the group treated with HCQ in comparison with 21.7% death in non-treated fetuses (Izmirly et al., 2012; Izmirly et al., 2010). Based on these results, a prospective trial called the “Preventative approach to congenital heart block with HCQ (PATCH)” study was launched to evaluate the role for HCQ in the prevention of CHB in pregnant women with a previous CHB child. Only one of 19 fetuses developed CCHB in Stage 1 of the trial, and Stage II testing is underway. HCQ interferes with lysosomal metabolism and may affect retinal development and so this trial is also further evaluating HCQ safety in utero (http://grantome.com/grant/NIH/R01-HD079951-01A1).
4. Historical Attempts at Fetal Pacing
Patients who are refractory to standard medical therapy for CCHB have few therapeutic options especially when fetal hydrops develops at a time when risks of delivery are high. Fetal pacing has the potential to increase the fetal heart rate and therefor augment cardiac output. Initial studies of chronic epicardial ventricular pacing in fetal sheep with CCHB showed that pacing was well tolerated and potentially a safe therapeutic option (Liddicoat et al., 1997). A number of attempts to pace fetuses with CCHB have been described, both via open uterus procedures as well as by approaches generally considered to be minimally invasive. The first attempts used minimally invasive techniques to place pacing leads either directly into the ventricle (Assad et al., 2003; Carpenter et al., 1986) or guided through the umbilical vein to the right ventricle (Walkinshaw, Welch, McCormack, & Walsh, 1994). These leads were attached to an external pacemaker. All three studies successfully paced the ventricle for short periods of time ranging from 4–36 hours, but failed secondary to lead dislodgement and complications of lead placement such as pericardial effusion (Assad et al., 2003; Carpenter et al., 1986; Walkinshaw et al., 1994) (Table 1).
Table 1:
Fetal Pacing Outcomes
| Study | Year | Pacing strategy | Time Paced | Outcomes |
|---|---|---|---|---|
| Carpenter et al | 1986 | Bipolar pigtail pacing catheter placed directly into RV via 19G needle | 4 hrs and 15 min | Fetal demise for unknown reasons. Autopsy revealed pericardial effusion. |
| Walkinshaw et al | 1994 | Teflon coated pacing lead guided through fetal umbilical vein and guided through IVC and RA into RV | 8 hrs | Initial lead dislodgement. Fetal demise possibly secondary to injury to myocardium during second attempted lead placement. |
| Silverman et al | 1998 | Direct epicardial pacemaker placement in fetus | Unknown | Fetal demise from surgical complications |
| Assad et al | 2003 | 18G needle used to place lead directly into LV myocardium and 2nd lead in thoracic wall with pacemaker in maternal abdominal wall | 36 hrs | Fetal demise possibly secondary to large pericardial effusion likely from lead placement complication. |
| Eghtesady et al | 2011 | Open fetal surgery for placement of unipolar pacemaker | 5 days | Loss of communication with pacemaker and inability to increase rate. Fetal demise from chronic multi-organ failure |
| Cuneo et al | 2017 | “EXIT” ex utero intrapartum treatment. Uterus opened and temporary epicardial RV pacing wire placed into RV via subxiphoid incision | 3 days | Successfully delivered and transitioned to permanent pacemaker on DOL3 |
Invasive approaches to placement of pacing leads include open fetal surgery for patients with CCHB who were refractory to medical therapy. In the first attempt at fetal pacing performed via open surgery for direct epicardial pacemaker implantation, a unipolar pacemaker was successfully implanted in a fetus with CCHB suffering from fetal hydrops and multi-organ failure via open fetal surgery. Pacing was successful for 5 days prior to demise secondary to end organ damage. This study suggested that increasing the fetal HR indeed increased cardiac output and that continuing augmentation of the cardiac output could potentially reverse end-organ damage; however, there was loss of communication with the device post implantation and this possibility could not be proven (Eghtesady et al., 2011). More recently, a novel ex utero intrapartum treatment (EXIT) was performed on a 36-week fetus exposed via uterine incision. A temporary epicardial pacing wire was sutured into the RV through a subxyphoid approach. The umbilical cord was then clamped, and the patient was delivered immediately with a permanent pacemaker placed on the third day of life (Cuneo, Mitchell, et al., 2017) (Table 1). Invasive surgical placement of fetal pacemakers comes with significant surgical and medical risks, but these studies encouragingly demonstrate that fetal pacing is possible. They also support the hope that early intervention prior to severe end-organ damage might allow for reversal of disease and thus decrease the risk of in utero demise.
5. Novel Fetal Pacing Therapies
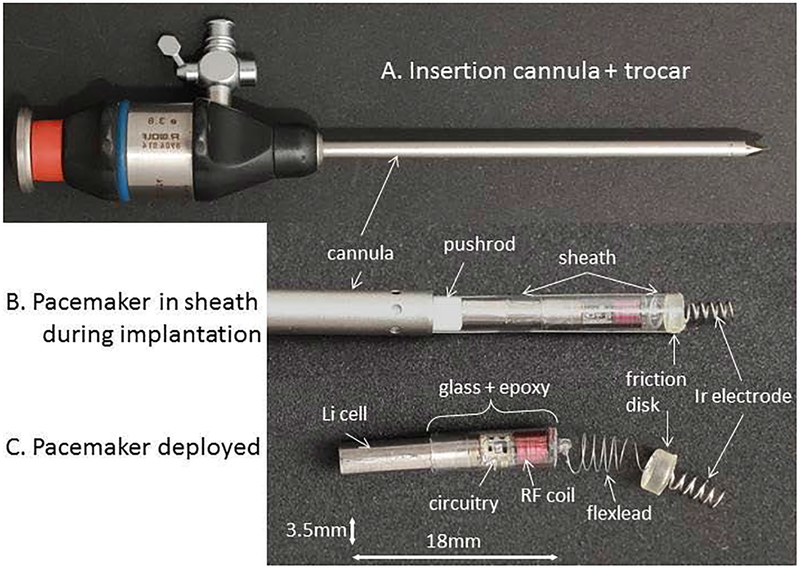
A potential therapy on the horizon for fetal pacing in CCHB is a self-contained percutaneously implantable micropacemaker developed by our group. The complete implanted device is 3.48 mm in diameter and 2.4 cm in length. It includes an encapsulated circuit board, corkscrew electrode tip, flexible lead, and lithium power cell. The device paces in a fixed rate VOO mode at 100–110 bpm. An inductive recharging system further allows the lithium cell to be recharged in utero until delivery via a transmitting coil placed over the mother’s abdomen (Loeb et al., 2013; Zhou et al., 2014). This recharging capacity allows the pacemaker to be far smaller than other miniaturized pacemakers being developed for adults and large children. These other miniaturized pacemakers, known as “leadless” pacemakers, are generally used in adults and are delivered percutaneously through the femoral vein to the apex of the right ventricle. While the utility of these leadless pacemakers has been demonstrated in adults, they are 5–7 times larger than the fetal micropacemaker and use a lead configuration that cannot be extrapolated to the fetus (Tjong & Reddy, 2017). The fetal micropacemaker is held in a sheath and implanted through a surgical trocar via subxiphoid epicardial approach (Figure 4). The micropacemaker was first validated in adult rabbits hearts (Loeb et al., 2013) and then optimized through trials in pregnant ewes with successful capture in the final four sheep fetuses (Bar-Cohen et al., 2015). A somewhat larger version of the fetal micropacemaker has been further adapted for pediatric use and successfully deployed into the pericardial space via the subxiphoid approach in an adult pig model (Bar-Cohen et al., 2018). In the near future, such minimally invasive pericardial pacing could offer a definitive treatment for CHB in both fetus and infant and might offer another pacing option for pediatric and adult patient populations for whom a conventional pacemaker or intraventricular leadless pacemaker is not an option. The fetal micropacemaker devices will be studied under a Humanitarian Use Designation (HUD) from the Food and Drug Administration, and the first human implant is expected soon.
Figure 4:
Fetal micropacemaker and its implantation system. (a): Implantation cannula (4.5 mm o.d. × 3.8 mm i.d.) with sharpened tip of the trocar protruding from the tip. (b): Configuration during implantation with the micropacemaker inside a polyimide plastic sheath that slides through the cannula. The friction disk is wedged into the end of the sheath, allowing the protruding Ir electrode to be turned into the myocardium (pre-deployment). The pacemaker is deployed from the sheath by a pushrod after confirming ventricular capture by ultrasound. (c): The fetal pacemaker as deployed. Features from left to right: battery case of lithium cell, which functions as return electrode; glass sleeve for epoxy encapsulation; printed circuit board with discrete surface-mount circuitry and RF coil for inductive recharging; flexible lead (75 μm Pt-30Ir with Parylene-C insulation; epoxy friction disk over welded joint, corkscrew electrode (254 μm Ir with Parylene-C insulation).
6. Conclusions
CCHB is a life-threatening medical condition without proven therapies. Current medical therapies aimed at decreasing inflammation through the maternal administration of anti-inflammatory treatments and increasing fetal cardiac output through maternal administration of beta-agonists have shown, at most, only marginal benefits (Jaeggi et al., 2004). These anti-inflammatory therapies cannot reverse CCHB although they may help decrease myocarditis and the development of cardiomyopathy (Cuneo et al., 2016). Advances in diagnosis and introduction of strict surveillance protocols with early delivery indications have demonstrated small improvements in morbidity and mortality, but are still woefully inadequate (Cuneo et al., 2010). Ambulatory surveillance programs and wearable fetal heart rate monitors may hold the key to early identification of fetal bradyarrhythmias and evolving fetal heart block allowing for emergent treatment for potential conversion back to 1:1 conduction (Cuneo et al., 2016; Graatsma et al., 2009; Narayan et al., 2015). There is also preliminary data suggesting a role for prophylactic treatment with HCQ, but the study is still in process and the safety profile of the medication needs to be further evaluated (Izmirly et al., 2012; Izmirly et al., 2010). To date, intrauterine fetal pacing has not been successful potentially due to the high-risk invasive placement techniques and problems with lead dislodgement. The development of a fully implantable micropacemaker via a minimally invasive approach may hold the potential to pace fetal patients with CCHB and reverse fetal hydrops. This could allow for survival to a full-term delivery and change CCHB from one of the most dangerous fetal arrhythmias to one that can be successfully managed in utero until permanent pacemaker placement postnatally.
Sources of Funding:
This research was funded by a National Institutes of Health R01 grant (1R01HD075135), the Southern California Clinical and Translational Science Institute, the Coulter Foundation, and the L.K. Whittier Foundation.
Footnotes
Disclosures: Issued and pending patents assigned to the affiliated institutions and related to the fetal micropacemaker device and implantation methods and equipment.
References:
- Alvarez D, Briassouli P, Clancy RM, Zavadil J, Reed JH, Abellar RG, … Buyon JP (2011). A novel role of endothelin-1 in linking Toll-like receptor 7-mediated inflammation to fibrosis in congenital heart block. J Biol Chem, 286(35), 30444–30454. doi: 10.1074/jbc.M111.263657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assad RS, Zielinsky P, Kalil R, Lima G, Aramayo A, Santos A, … Oliveira SA (2003). New lead for in utero pacing for fetal congenital heart block. J Thorac Cardiovasc Surg, 126(1), 300–302. [DOI] [PubMed] [Google Scholar]
- Bar-Cohen Y, Loeb GE, Pruetz JD, Silka MJ, Guerra C, Vest AN, … Chmait RH (2015). Preclinical testing and optimization of a novel fetal micropacemaker. Heart Rhythm, 12(7), 1683–1690. doi: 10.1016/j.hrthm.2015.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Cohen Y, Silka MJ, Hill AC, Pruetz JD, Chmait RH, Zhou L, … Loeb GE (2018). Minimally Invasive Implantation of a Micropacemaker Into the Pericardial Space. Circ Arrhythm Electrophysiol, 11(7), e006307. doi: 10.1161/CIRCEP.118.006307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baruteau AE, Behaghel A, Fouchard S, Mabo P, Schott JJ, Dina C, … Probst V (2012). Parental electrocardiographic screening identifies a high degree of inheritance for congenital and childhood nonimmune isolated atrioventricular block. Circulation, 126(12), 1469–1477. doi: 10.1161/CIRCULATIONAHA.111.069161 [DOI] [PubMed] [Google Scholar]
- Baruteau AE, Fouchard S, Behaghel A, Mabo P, Villain E, Thambo JB, … Probst V (2012). Characteristics and long-term outcome of non-immune isolated atrioventricular block diagnosed in utero or early childhood: a multicentre study. Eur Heart J, 33(5), 622–629. doi: 10.1093/eurheartj/ehr347 [DOI] [PubMed] [Google Scholar]
- Baruteau AE, Pass RH, Thambo JB, Behaghel A, Le Pennec S, Perdreau E, … McLeod CJ (2016). Congenital and childhood atrioventricular blocks: pathophysiology and contemporary management. Eur J Pediatr, 175(9), 1235–1248. doi: 10.1007/s00431-016-2748-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baruteau AE, Probst V, & Abriel H (2015). Inherited progressive cardiac conduction disorders. Curr Opin Cardiol, 30(1), 33–39. doi: 10.1097/HCO.0000000000000134 [DOI] [PubMed] [Google Scholar]
- Brito-Zerón P, Izmirly PM, Ramos-Casals M, Buyon JP, & Khamashta MA (2015). The clinical spectrum of autoimmune congenital heart block. Nat Rev Rheumatol, 11(5), 301–312. doi: 10.1038/nrrheum.2015.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brucato A, Cimaz R, Caporali R, Ramoni V, & Buyon J (2011). Pregnancy outcomes in patients with autoimmune diseases and anti-Ro/SSA antibodies. Clin Rev Allergy Immunol, 40(1), 27–41. doi: 10.1007/s12016-009-8190-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brucato A, Jonzon A, Friedman D, Allan LD, Vignati G, Gasparini M, … Buyon J (2003). Proposal for a new definition of congenital complete atrioventricular block. Lupus, 12(6), 427–435. doi: 10.1191/0961203303lu408oa [DOI] [PubMed] [Google Scholar]
- Budin N, & Abboud S (1994). Real-time multichannel abdominal fetal ECG monitor using digital signal coprocessor. Comput Biol Med, 24(6), 451–462. [DOI] [PubMed] [Google Scholar]
- Buyon JP, Hiebert R, Copel J, Craft J, Friedman D, Katholi M, … Skovron ML (1998). Autoimmune-associated congenital heart block: demographics, mortality, morbidity and recurrence rates obtained from a national neonatal lupus registry. J Am Coll Cardiol, 31(7), 1658–1666. [DOI] [PubMed] [Google Scholar]
- Carpenter RJ, Strasburger JF, Garson A, Smith RT, Deter RL, & Engelhardt HT (1986). Fetal ventricular pacing for hydrops secondary to complete atrioventricular block. J Am Coll Cardiol, 8(6), 1434–1436. [DOI] [PubMed] [Google Scholar]
- Ciardulli A, D’Antonio F, Magro-Malosso ER, Manzoli L, Anisman P, Saccone G, & Berghella V (2018). Maternal steroid therapy for fetuses with second-degree immune-mediated congenital atrioventricular block: a systematic review and meta-analysis. Acta Obstet Gynecol Scand, 97(7), 787–794. doi: 10.1111/aogs.13338 [DOI] [PubMed] [Google Scholar]
- Ciardulli A, D’Antonio F, Magro-Malosso ER, Saccone G, Manzoli L, Radolec M, & Berghella V (2017). Maternal steroid therapy for fetuses with immune-mediated complete atrioventricular block: a systematic review and meta-analysis. J Matern Fetal Neonatal Med, 1–261. doi: 10.1080/14767058.2017.1419182 [DOI] [PubMed] [Google Scholar]
- Clancy RM, Alvarez D, Komissarova E, Barrat FJ, Swartz J, & Buyon JP (2010). Ro60-associated single-stranded RNA links inflammation with fetal cardiac fibrosis via ligation of TLRs: a novel pathway to autoimmune-associated heart block. J Immunol, 184(4), 2148–2155. doi: 10.4049/jimmunol.0902248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costedoat-Chalumeau N, Amoura Z, Le Thi Hong D, Wechsler B, Vauthier D, Ghillani P, … Piette JC (2003). Questions about dexamethasone use for the prevention of anti-SSA related congenital heart block. Ann Rheum Dis, 62(10), 1010–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuneo BF, Ambrose SE, & Tworetzky W (2016). Detection and successful treatment of emergent anti-SSA-mediated fetal atrioventricular block. Am J Obstet Gynecol, 215(4), 527–528. doi: 10.1016/j.ajog.2016.07.002 [DOI] [PubMed] [Google Scholar]
- Cuneo BF, Fruitman D, Benson DW, Ngan BY, Liske MR, Wahren-Herlineus M, … Jaeggi E (2011). Spontaneous rupture of atrioventricular valve tensor apparatus as late manifestation of anti-Ro/SSA antibody-mediated cardiac disease. Am J Cardiol, 107(5), 761–766. doi: 10.1016/j.amjcard.2010.10.059 [DOI] [PubMed] [Google Scholar]
- Cuneo BF, Lee M, Roberson D, Niksch A, Ovadia M, Parilla BV, & Benson DW (2010). A management strategy for fetal immune-mediated atrioventricular block. J Matern Fetal Neonatal Med, 23(12), 1400–1405. doi: 10.3109/14767051003728237 [DOI] [PubMed] [Google Scholar]
- Cuneo BF, Mitchell MB, Marwan AI, Green M, von Alvensleben JC, Reynolds R, … Galan HL (2017). Ex utero Intrapartum Treatment to Ventricular Pacing: A Novel Delivery Strategy for Complete Atrioventricular Block with Severe Bradycardia. Fetal Diagn Ther, 42(4), 311–314. doi: 10.1159/000475815 [DOI] [PubMed] [Google Scholar]
- Cuneo BF, Moon-Grady AJ, Sonesson SE, Levasseur S, Hornberger L, Donofrio MT, … Jaeggi E (2017). Heart sounds at home: feasibility of an ambulatory fetal heart rhythm surveillance program for anti-SSA-positive pregnancies. J Perinatol, 37(3), 226–230. doi: 10.1038/jp.2016.220 [DOI] [PubMed] [Google Scholar]
- Cuneo BF, Sonesson SE, Levasseur S, Moon-Grady AJ, Krishnan A, Donofrio MT, … Jaeggi E (2018). Home Monitoring for Fetal Heart Rhythm During Anti-Ro Pregnancies. J Am Coll Cardiol, 72(16), 1940–1951. doi: 10.1016/j.jacc.2018.07.076 [DOI] [PubMed] [Google Scholar]
- Dancea A, Fouron JC, Miró J, Skoll A, & Lessard M (2000). Correlation between electrocardiographic and ultrasonographic time-interval measurements in fetal lamb heart. Pediatr Res, 47(3), 324–328. [DOI] [PubMed] [Google Scholar]
- Donofrio MT, Moon-Grady AJ, Hornberger LK, Copel JA, Sklansky MS, Abuhamad A, … American Heart Association Adults With Congenital Heart Disease Joint Committee of the Council on Cardiovascular Disease in the Young and Council on Clinical Cardiology, C. u. o. C. S. a. A. (2014). Diagnosis and treatment of fetal cardiac disease: a scientific statement from the American Heart Association. Circulation, 129(21), 2183–2242. doi: 10.1161/01.cir.0000437597.44550.5d [DOI] [PubMed] [Google Scholar]
- Eghtesady P, Michelfelder EC, Knilans TK, Witte DP, Manning PB, & Crombleholme TM (2011). Fetal surgical management of congenital heart block in a hydropic fetus: lessons learned from a clinical experience. J Thorac Cardiovasc Surg, 141(3), 835–837. doi: 10.1016/j.jtcvs.2010.06.048 [DOI] [PubMed] [Google Scholar]
- El-Haieg DO, Zanati MF, & El-Foual FM (2007). Plasmapheresis and pregnancy outcome in patients with antiphospholipid syndrome. Int J Gynaecol Obstet, 99(3), 236–241. doi: 10.1016/j.ijgo.2007.05.045 [DOI] [PubMed] [Google Scholar]
- Eliasson H, Sonesson SE, Sharland G, Granath F, Simpson JM, Carvalho JS, … Cardiology, F. W. G. o. t. E. A. o. P. (2011). Isolated atrioventricular block in the fetus: a retrospective, multinational, multicenter study of 175 patients. Circulation, 124(18), 1919–1926. doi: 10.1161/CIRCULATIONAHA.111.041970 [DOI] [PubMed] [Google Scholar]
- Friedman D, Duncanson L, Glickstein J, & Buyon J (2003). A review of congenital heart block. Images Paediatr Cardiol, 5(3), 36–48. [PMC free article] [PubMed] [Google Scholar]
- Friedman DM, Llanos C, Izmirly PM, Brock B, Byron J, Copel J, … Buyon JP (2010). Evaluation of fetuses in a study of intravenous immunoglobulin as preventive therapy for congenital heart block: Results of a multicenter, prospective, open-label clinical trial. Arthritis Rheum, 62(4), 1138–1146. doi: 10.1002/art.27308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyfe DA, Meyer KB, & Case CL (1993). Sonographic assessment of fetal cardiac arrhythmias. Semin Ultrasound CT MR, 14(4), 286–297. [DOI] [PubMed] [Google Scholar]
- Graatsma EM, Jacod BC, van Egmond LA, Mulder EJ, & Visser GH (2009). Fetal electrocardiography: feasibility of long-term fetal heart rate recordings. BJOG, 116(2), 334–337; discussion 337–338. doi: 10.1111/j.1471-0528.2008.01951.x [DOI] [PubMed] [Google Scholar]
- Gürcan HM, Keskin DB, & Ahmed AR (2010). Information for healthcare providers on general features of IGIV with emphasis on differences between commercially available products. Autoimmun Rev, 9(8), 553–559. doi: 10.1016/j.autrev.2010.03.003 [DOI] [PubMed] [Google Scholar]
- Hornberger LK, & Collins K (2008). New insights into fetal atrioventricular block using fetal magnetocardiography. J Am Coll Cardiol, 51(1), 85–86. doi: 10.1016/j.jacc.2007.09.016 [DOI] [PubMed] [Google Scholar]
- Izmirly PM, Costedoat-Chalumeau N, Pisoni CN, Khamashta MA, Kim MY, Saxena A, … Buyon JP (2012). Maternal use of hydroxychloroquine is associated with a reduced risk of recurrent anti-SSA/Ro-antibody-associated cardiac manifestations of neonatal lupus. Circulation, 126(1), 76–82. doi: 10.1161/CIRCULATIONAHA.111.089268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izmirly PM, Halushka MK, Rosenberg AZ, Whelton S, Rais-Bahrami K, Nath DS, … Buyon JP (2017). Clinical and pathologic implications of extending the spectrum of maternal autoantibodies reactive with ribonucleoproteins associated with cutaneous and now cardiac neonatal lupus from SSA/Ro and SSB/La to U1RNP. Autoimmun Rev, 16(9), 980–983. doi: 10.1016/j.autrev.2017.07.013 [DOI] [PubMed] [Google Scholar]
- Izmirly PM, Kim MY, Llanos C, Le PU, Guerra MM, Askanase AD, … Buyon JP (2010). Evaluation of the risk of anti-SSA/Ro-SSB/La antibody-associated cardiac manifestations of neonatal lupus in fetuses of mothers with systemic lupus erythematosus exposed to hydroxychloroquine. Ann Rheum Dis, 69(10), 1827–1830. doi: 10.1136/ard.2009.119263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izmirly PM, Saxena A, Kim MY, Wang D, Sahl SK, Llanos C, … Buyon JP (2011). Maternal and fetal factors associated with mortality and morbidity in a multi-racial/ethnic registry of anti-SSA/Ro-associated cardiac neonatal lupus. Circulation, 124(18), 1927–1935. doi: 10.1161/CIRCULATIONAHA.111.033894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeggi ET, Fouron JC, Silverman ED, Ryan G, Smallhorn J, & Hornberger LK (2004). Transplacental fetal treatment improves the outcome of prenatally diagnosed complete atrioventricular block without structural heart disease. Circulation, 110(12), 1542–1548. doi: 10.1161/01.CIR.0000142046.58632.3A [DOI] [PubMed] [Google Scholar]
- Jaeggi ET, Hamilton RM, Silverman ED, Zamora SA, & Hornberger LK (2002). Outcome of children with fetal, neonatal or childhood diagnosis of isolated congenital atrioventricular block. A single institution’s experience of 30 years. J Am Coll Cardiol, 39(1), 130–137. [DOI] [PubMed] [Google Scholar]
- Lafyatis R, York M, & Marshak-Rothstein A (2006). Antimalarial agents: closing the gate on Toll-like receptors? Arthritis Rheum, 54(10), 3068–3070. doi: 10.1002/art.22157 [DOI] [PubMed] [Google Scholar]
- Liddicoat JR, Klein JR, Reddy VM, Klautz RJ, Teitel DF, & Hanley FL (1997). Hemodynamic effects of chronic prenatal ventricular pacing for the treatment of complete atrioventricular block. Circulation, 96(3), 1025–1030. [DOI] [PubMed] [Google Scholar]
- Loeb GE, Zhou L, Zheng K, Nicholson A, Peck RA, Krishnan A, … Bar-Cohen Y (2013). Design and testing of a percutaneously implantable fetal pacemaker. Ann Biomed Eng, 41(1), 17–27. doi: 10.1007/s10439-012-0631-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino S, Yonemoto H, Itoh S, & Takeda S (2007). Effect of steroid administration and plasmapheresis to prevent fetal congenital heart block in patients with systemic lupus erythematosus and/or Sjögren’s syndrome. Acta Obstet Gynecol Scand, 86(9), 1145–1146. doi: 10.1080/00016340701343024 [DOI] [PubMed] [Google Scholar]
- Makita N, Seki A, Sumitomo N, Chkourko H, Fukuhara S, Watanabe H, … Delmar M (2012). A connexin40 mutation associated with a malignant variant of progressive familial heart block type I. Circ Arrhythm Electrophysiol, 5(1), 163–172. doi: 10.1161/CIRCEP.111.967604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaëlsson M, & Engle MA (1972). Congenital complete heart block: an international study of the natural history. Cardiovasc Clin, 4(3), 85–101. [PubMed] [Google Scholar]
- Modi N, Lewis H, Al-Naqeeb N, Ajayi-Obe M, Doré CJ, & Rutherford M (2001). The effects of repeated antenatal glucocorticoid therapy on the developing brain. Pediatr Res, 50(5), 581–585. doi: 10.1203/00006450-200111000-00008 [DOI] [PubMed] [Google Scholar]
- Narayan HK, Vignola EF, Fifer WP, & Williams IA (2015). Assessment of Cardiac Rate and Rhythm in Fetuses with Arrhythmia via Maternal Abdominal Fetal Electrocardiography. AJP Rep, 5(2), e176–182. doi: 10.1055/s-0035-1558401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perricone R, De Carolis C, Kröegler B, Greco E, Giacomelli R, Cipriani P, … Perricone C (2008). Intravenous immunoglobulin therapy in pregnant patients affected with systemic lupus erythematosus and recurrent spontaneous abortion. Rheumatology (Oxford), 47(5), 646–651. doi: 10.1093/rheumatology/ken046 [DOI] [PubMed] [Google Scholar]
- Pisoni CN, Brucato A, Ruffatti A, Espinosa G, Cervera R, Belmonte-Serrano M, … Khamashta MA (2010). Failure of intravenous immunoglobulin to prevent congenital heart block: Findings of a multicenter, prospective, observational study. Arthritis Rheum, 62(4), 1147–1152. doi: 10.1002/art.27350 [DOI] [PubMed] [Google Scholar]
- Rein AJ, Mevorach D, Perles Z, Gavri S, Nadjari M, Nir A, & Elchalal U (2009). Early diagnosis and treatment of atrioventricular block in the fetus exposed to maternal anti-SSA/Ro-SSB/La antibodies: a prospective, observational, fetal kinetocardiogram-based study. Circulation, 119(14), 1867–1872. doi: 10.1161/CIRCULATIONAHA.108.773143 [DOI] [PubMed] [Google Scholar]
- Rein AJ, O’Donnell C, Geva T, Nir A, Perles Z, Hashimoto I, … Sahn DJ (2002). Use of tissue velocity imaging in the diagnosis of fetal cardiac arrhythmias. Circulation, 106(14), 1827–1833. [DOI] [PubMed] [Google Scholar]
- Rosenthal E, Gordon PA, Simpson JM, & Sharland GK (2005). Letter regarding article by Jaeggi et al, “transplacental fetal treatment improves the outcome of prenatally diagnosed complete atrioventricular block without structural heart disease”. Circulation, 111(18), e287–288; author reply e287–288. doi: 10.1161/01.CIR.0000164275.69617.B2 [DOI] [PubMed] [Google Scholar]
- Ruffatti A, Cerutti A, Favaro M, Del Ross T, Calligaro A, Hoxha A, … Milanesi O (2016). Plasmapheresis, intravenous immunoglobulins and bethametasone - a combined protocol to treat autoimmune congenital heart block: a prospective cohort study. Clin Exp Rheumatol, 34(4), 706–713. [PubMed] [Google Scholar]
- Ruffatti A, Milanesi O, Chiandetti L, Cerutti A, Gervasi MT, De Silvestro G, … Punzi L (2012). A combination therapy to treat second-degree anti-Ro/La-related congenital heart block: a strategy to avoid stable third-degree heart block? Lupus, 21(6), 666–671. doi: 10.1177/0961203311430969 [DOI] [PubMed] [Google Scholar]
- Schmidt KG, Ulmer HE, Silverman NH, Kleinman CS, & Copel JA (1991). Perinatal outcome of fetal complete atrioventricular block: a multicenter experience. J Am Coll Cardiol, 17(6), 1360–1366. [DOI] [PubMed] [Google Scholar]
- Schott JJ, Alshinawi C, Kyndt F, Probst V, Hoorntje TM, Hulsbeek M, … Le Marec H (1999). Cardiac conduction defects associate with mutations in SCN5A. Nat Genet, 23(1), 20–21. doi: 10.1038/12618 [DOI] [PubMed] [Google Scholar]
- Strasburger JF, & Wakai RT (2010). Fetal cardiac arrhythmia detection and in utero therapy. Nat Rev Cardiol, 7(5), 277–290. doi: 10.1038/nrcardio.2010.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjong FV, & Reddy VY (2017). Permanent Leadless Cardiac Pacemaker Therapy: A Comprehensive Review. Circulation, 135(15), 1458–1470. doi: 10.1161/CIRCULATIONAHA.116.025037 [DOI] [PubMed] [Google Scholar]
- Tran HB, Cavill D, Buyon JP, & Gordon TP (2004). Intravenous immunoglobulin and placental transport of anti-Ro/La antibodies: comment on the letter by Kaaja and Julkunen. Arthritis Rheum, 50(1), 337–338; author reply 338. doi: 10.1002/art.11498 [DOI] [PubMed] [Google Scholar]
- Wacker-Gussmann A, Strasburger JF, Cuneo BF, & Wakai RT (2014). Diagnosis and treatment of fetal arrhythmia. Am J Perinatol, 31(7), 617–628. doi: 10.1055/s-0034-1372430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walkinshaw SA, Welch CR, McCormack J, & Walsh K (1994). In utero pacing for fetal congenital heart block. Fetal Diagn Ther, 9(3), 183–185. doi: 10.1159/000263929 [DOI] [PubMed] [Google Scholar]
- Whitelaw A, & Thoresen M (2000). Antenatal steroids and the developing brain. Arch Dis Child Fetal Neonatal Ed, 83(2), F154–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Cuneo BF, Strasburger JF, Huhta JC, Gotteiner NL, & Wakai RT (2008). Electrophysiological characteristics of fetal atrioventricular block. J Am Coll Cardiol, 51(1), 77–84. doi: 10.1016/j.jacc.2007.06.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Vest AN, Chmait RH, Bar-Cohen Y, Pruetz J, Silka M, … Loeb GE (2014). A percutaneously implantable fetal pacemaker. Conf Proc IEEE Eng Med Biol Soc, 2014, 4459–4463. doi: 10.1109/EMBC.2014.6944614 [DOI] [PMC free article] [PubMed] [Google Scholar]