Figure 2.
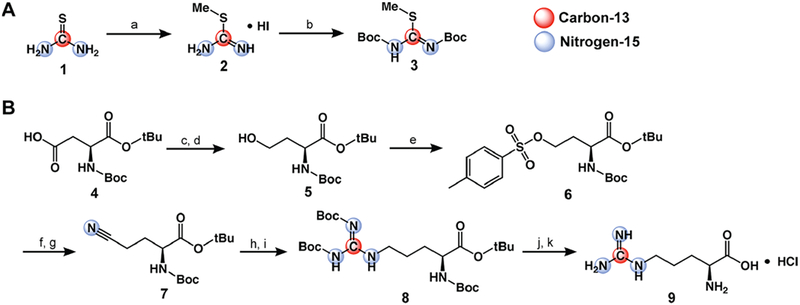
Synthesis of [6-13C,15N3]-arginine. (A) Synthetic scheme for Boc-protected thiourea-derived precursor (compound 3). (a) 1.15 equiv of CH3I, EtOH, reflux, 3 h; (b) 4 equiv of Boc2O, DCM, sat. NaHCO3, RT, 5 d. (B) Synthetic scheme for [6-13C,15N3]-arginine. (c) 1.5 equiv of ECF, 1.5 equiv of Et3N, THF, −10 °C → RT, 30 min; (d) 2.1 equiv of NaBH4, THF/H2O, 0 °C → RT, 1 h; (e) 2 equiv of TsCl, 5 equiv of Et3N, 0.1 equiv of DMAP, DCM, 0 °C → RT, 1 h; (f) 2 equiv of NaI, acetone, reflux, 1 h; (g) 1.2 equiv of KC15N, DMSO, 80 °C, 18 h; (h) H2, Pd/C, AcOH, RT, 2 h; (i) 1 equiv of compound 3, 5 equiv of Et3N, DMSO, RT, 24 h; (j) 1:9 TFA/DCM, RT, 18 h; (k) 1 M HCl.
