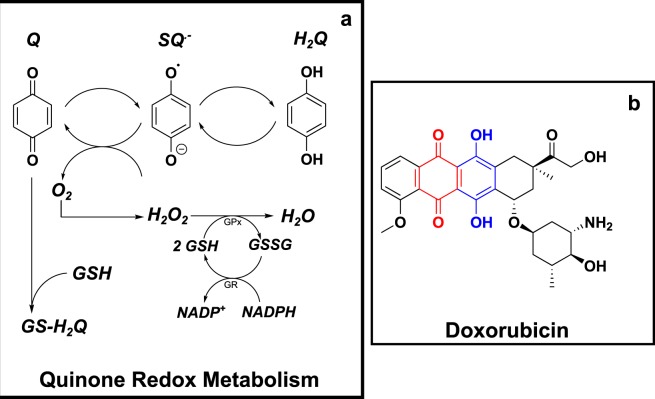
Figure 1.
(a) Quinone redox cycling, ROS formation and GSH-based detoxification. A schematic of the single electron reduction of a quinone (Q) to a semiquinone radical anion (SQ.−), followed by complete reduction to the hydroquinone (H2Q). The figure shows the concomitant reduction of molecular oxygen by SQ.− to form the ROS, superoxide (O2.−), followed by its dismutation into hydrogen peroxide (H2O2), which is detoxified by glutathione (GSH) into harmless H2O through the glutathione peroxidase (GPx) reaction. GSH is regenerated from its oxidised form (GSSG), catalysed by the glutathione reduction (GR) reaction. Finally, the glutathione-quinone adduct (GS-H2Q) formation represents the reductive addition (Michael reaction) between GSH and the Q electrophile. (b) Chemical structure of doxorubicin. The anthracycline contains both the quinone (red) and hydroquinone (blue) moieties within its chemical structure. The hydroquinone is the site of auto-oxidation for doxorubicin.