Abstract
Background
Chronic kidney disease is a recognized independent risk factor for cardiovascular disease, but whether the risks of ST‐segment–elevation myocardial infarction (STEMI) and non–ST‐segment–elevation myocardial infarction (NSTEMI) differ in the chronic kidney disease population is unknown.
Methods and Results
Using administrative data from Ontario, Canada, we examined patients ≥66 years of age with an outpatient estimated glomerular filtration rate (eGFR) and albuminuria measure for incident myocardial infarction from 2002 to 2015. Adjusted Fine and Gray subdistribution hazard models accounting for the competing risk of death were used. In 248 438 patients with 1.2 million person‐years of follow‐up, STEMI, NSTEMI, and death occurred in 1436 (0.58%), 4431 (1.78%), and 30 015 (12.08%) patients, respectively. The highest level of albumin‐to‐creatinine ratio (>30 mg/mmol) was associated with a 2‐fold higher adjusted risk of both STEMI and NSTEMI among patients with eGFR≥60 mL/(min·1.73 m2) compared to albumin‐to‐creatinine ratio <3 mg/mmol. The lowest level of eGFR (<30 mL/[min·1.73 m2]) was not associated with higher STEMI risk but with a 4‐fold higher risk of NSTEMI compared to those with eGFR≥60 mL/(min·1.73 m2). The lowest eGFR (<30 mL/[min·1.73 m2]) and highest albumin‐to‐creatinine ratio (>30 mg/mmol) were associated with a greater than 4‐fold higher risk of both STEMI and NSTEMI (subdistribution hazard models [95% confidence interval] 4.53 [3.30‐6.21] and 4.42 [3.67‐5.32], respectively) compared to albumin‐to‐creatinine ratio <3 mg/mmol and eGFR≥60 mL/(min·1.73 m2).
Conclusions
Elevations in albuminuria are associated with a higher risk of both NSTEMI and STEMI, regardless of kidney function, whereas reduced kidney function alone is associated with a higher NSTEMI risk.
Keywords: chronic kidney disease, competing risks, epidemiology, myocardial infarction, non–ST‐segment–elevation acute coronary syndrome, ST‐segment–elevation myocardial infarction
Subject Categories: Epidemiology, Risk Factors, Myocardial Infarction
Clinical Perspective
What Is New?
This large, epidemiological study demonstrates that elevations in albuminuria were associated with a higher risk of both non–ST‐elevation myocardial infarction (MI) and ST‐elevation MI, regardless of kidney function, whereas reduced kidney function alone is associated with a higher non–ST‐elevation MI risk.
What Are the Clinical Implications?
Because patients with chronic kidney disease are at a very high risk of MI, preceding knowledge of a patient's estimated glomerular filtration rate and albuminuria may aid in predicting the risk of an ST‐elevation MI versus a non–ST‐elevation MI.
Chronic kidney disease (CKD), defined by declines in the estimated glomerular filtration rate (eGFR) and/or the presence of albuminuria (measured by the albumin‐to‐creatinine ratio [ACR]), is highly prevalent (12% to 14%) in developed nations and is anticipated to rise over the coming decades.1, 2, 3 Among patients with CKD, cardiovascular disease is the leading cause of death with the adjusted 2‐year mortality of patients with acute myocardial infarction (MI) approaching 20%.4, 5, 6
The CKD population is heterogeneous in that a substantial proportion of patients present with isolated albuminuria, isolated reductions in kidney function, or both.1 The differential combinations of reductions in eGFR and/or elevations in ACR alter multiple physiological processes and may lead individuals to different MI types. Previous studies suggest that elevations in ACR lead to a prothrombotic state, predisposing individuals to ST‐segment–elevation myocardial infarction (STEMI), whereas declines in eGFR predispose individuals to vascular calcification and non–ST‐segment–elevation myocardial infarction (NSTEMI).
An understanding of the individual and combined contributions of albuminuria and kidney function may aid in accurately determining MI risk. This increased understanding can help clinicians to target appropriate primary preventative therapies for their patients with CKD. Early STEMI identification is of particular importance because the institution and success of reperfusion strategies are time dependent. However, CKD patients with a STEMI are nearly 2‐fold more likely to present with atypical features, leading to possible delays in treatments. As a result, the determination of STEMI risk based on eGFR and ACR levels may heighten suspicion and aid in identification of high‐risk individuals at a population level. It is well established that albuminuria and eGFR are associated with incident NSTEMI and MI‐induced mortality7, 8, 9; however, considerably less is known regarding the relationship between STEMI and CKD. It remains unclear whether simple, readily available measures of kidney function are similarly predictive of STEMI events. Thus, we set out to determine the association of MI type according to levels of albuminuria and eGFR. We hypothesized that the risk and types (STEMI and NSTEMI) of MI would differ by levels of kidney function.
Methods
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Study Design and Setting
We conducted a retrospective cohort study of individuals with an outpatient serum creatinine laboratory measurement and a random urine ACR between April 1, 2002 and March 31, 2013. Our cohort was followed for up to 3 years after the serum creatinine measurement. We used administrative databases held at the Institute for Clinical Evaluative Sciences (ICES) to obtain patient characteristics, laboratory, medication, and outcome data on residents of Ontario, Canada.10 These data sets were linked using unique encoded identifiers and analyzed at ICES. This study used individual‐level deterministic linkage across multiple databases to create our data set. Individuals were deidentified for analytic purposes, so informed consent was waived. The linkage rate for each of these databases was >96%. This study was approved by an institutional review committee at Sunnybrook Health Sciences Centre. The reporting of this study follows the RECORD (Reporting of Studies Conducted Using Observational Routinely Collected Health Data) guidelines for observational studies (Table S1).11
Participants
Patients were included in the study if they had a urine ACR measurement and an eGFR measurement within 12 months before the eGFR measurement. The Chronic Kidney Disease Epidemiology Collaboration equation was used to calculate eGFR.12 The date of the urine ACR measurement was counted as the index date for study inclusion. Outpatient eGFR measures have previously been validated.13 Serum creatinine values were corrected for isotope‐dilution mass spectrometry harmonization.
Individuals were excluded on the basis of the following criteria: (1) missing age, sex, ICES key number data, or non‐Ontario residents (data cleaning); (2) evidence of death on or before the index date; (3) age <66; (4) kidney transplant recipients; (5) evidence of chronic dialysis before index date; (6) history of MI, coronary artery bypass graft surgery, or percutaneous coronary intervention. Patients with age ≤66 were excluded; drug and medication information is captured in Ontario, Canada.
Exposures, Comorbidities, and Outcomes
At baseline, eGFR and ACR were used to categorize kidney function based on the KDIGO (Kidney Disease Improving Global Outcomes) guidelines.14 Outcomes were examined by combinations of ACR (<3, 3‐30, >30 mg/mmol) and eGFR (>60, 45‐59, 30‐44, <30 mL/[min·1.73 m2]) level as previous studies reported differential clinical risk based on the 2 combinations. KDIGO eGFR categories 15 to 29 and <15 were collapsed because of the small sample size.
Demographic variables were ascertained at index, including age, sex, income, place of residence, and clinical variables. Income was determined using the neighborhood‐level income based on an individual patient's postal code for his or her primary residence. Comorbidities were ascertained in the 5 years before the index date (angina, valve replacement, hypertension, diabetes mellitus, dyslipidemia, stroke or transient ischemic attack, and venous thromboembolism). Healthcare resource utilization was ascertained in the 1 year preceding the index date, including visits to hospitals, emergency departments, nephrologists, and cardiologists. We used the Adjusted Clinical Group scoring system to score comorbidity using The Johns Hopkins ACG System (version 10). The Adjusted Clinical Group is a population/patient case‐mix adjustment system that provides a relative measure of the individual's expected consumption of health services.15 International Classification of Diseases Revision 9 (ICD‐9) and 9‐CM (ICD‐9‐CM) codes are categorized into 32 groups, called ambulatory diagnostic groups, on the basis of clinical similarity, chronicity, likelihood of requiring specialty care, and disability. We also used resource utilization bands to ascertain resources utilization based on their overall disease burden. Medication prescription information was obtained up to 120 days before the index date.16
The primary study outcomes were STEMI hospitalization (ICD‐10‐CA codes I21.0‐3, R94.30, any diagnosis type) or NSTEMI hospitalization (ICD‐10 codes I21.4, R94.31, any diagnosis type). STEMI and NSTEMI ICD‐10 diagnostic codes have been validated with agreements of 85.2% and 100.0%, respectively, in those ≥65 years of age.17 If there were multiple eligible outcome events, we recorded only the first. We also examined all‐cause mortality defined using the death indicator in the Registered Persons Database.10 To account for the differences at baseline, we adjusted for demographics (age, sex, income quintile, long‐term care status), year of index, comorbid illness (angina, valve replacement, hypertension, diabetes mellitus, dyslipidemia, stroke or transient ischemic attack, atrial fibrillation or flutter, venous thromboembolism), healthcare utilization (number of visits to the hospital, emergency department, a nephrologist, a cardiologist, ambulatory diagnostic groups, and resource utilization bands), and medication usage (β‐blockers, antihypertensive agents, statins, antiplatelets, anticoagulants) in our models.
Statistical Methods
Baseline characteristics were estimated across categories of ACR, and differences were calculated using chi‐squared (categorical) and Kruskal‐Wallis (continuous) tests and are reported as P‐values. Significance was defined as P<0.05. Continuous data are presented as medians (25th, 75th percentiles), and categorical data as frequencies (percentages). We calculated the incidence rates (defined as the number of events per 100 000 person‐years of follow‐up) of STEMI and NSTEMI by eGFR and ACR categories. We examined the association of ACR and eGFR categories on the first event of STEMI or NSTEMI using the Fine‐Gray model to account for the competing event of death.18 This method allows the handling of multiple potential outcomes and is especially useful in CKD studies, where death is a common competing outcome.19 We utilized the subdistribution hazard ratio (sHR) which is a regression model for the cumulative incidence function. To examine if eGFR and ACR categories were effect modifiers on the association of STEMI and NSTEMI, additional models incorporating interaction terms were created (eGFR×ACR).19 All analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC).
Results
Population Demographics and Baseline Characteristics
In total, 3 798 652 patients had outpatient laboratory measurement records of serum creatinine and ACR, of whom 248 438 patients were included in the analytic cohort (Figure 1). Roughly one quarter of the study cohort had reduced kidney function (eGFR <60 mL/[min·1.73 m2]) and elevated albuminuria (≥3 mg/mmol), by 24% and 27%, respectively. The median (25th, 75th percentiles) age was 72±6 years across the entire cohort and was significantly different (P<0.001) among ACR categories (Table 1). The majority of the cohort were male (55.0%), but the percentage of women increased with increasing ACR. The proportion of individuals with all comorbidities including angina, hypertension, diabetes mellitus, and dyslipidemia increased across increasing ACR categories. This was also true for filled medication prescriptions for β‐blockers, antihypertensive agents, statins, antiplatelet agents, and anticoagulants.
Figure 1.
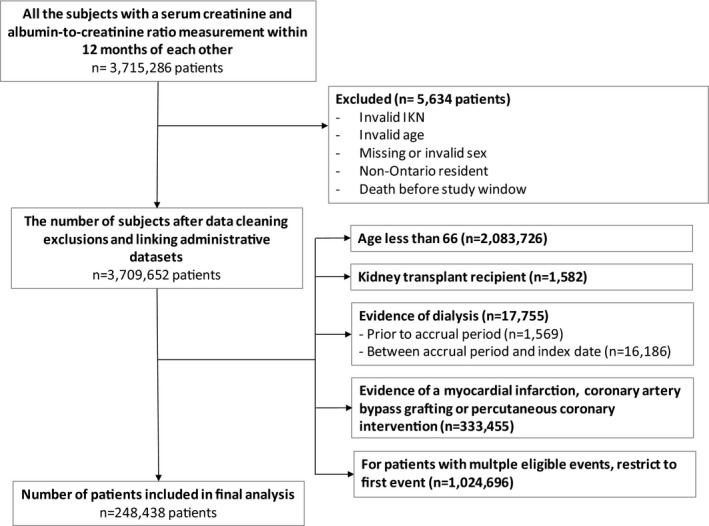
Cohort creation flowchart.
Table 1.
Baseline Characteristics Stratified by Albumin‐to‐Creatinine Ratio
| Total | Albumin‐to‐Creatinine Ratio (mg/mmol) | P Value | |||
|---|---|---|---|---|---|
| <3 | 3 to 30 | ≥30 | |||
| Total, N (%) | 248 438 | 183 522 (74) | 53 407 (21) | 11 509 (5) | |
| Age, median (25th, 75th percentiles) | 72 (67, 78) | 71 (67, 77) | 74 (68, 80) | 73 (68, 80) | <0.001 |
| Male, N (%) | 136 669 (55.0) | 103 122 (56.2) | 28 226 (52.9) | 5321 (46.2) | <0.001 |
| Income quintile, N (%) | |||||
| 1 | 48 585 (19.6) | 34 419 (18.8) | 11 497 (21.5) | 2669 (23.2) | <0.001 |
| 2 | 55 150 (22.2) | 40 129 (21.9) | 12 202 (22.8) | 2819 (24.5) | |
| 3 | 49 990 (20.1) | 37 113 (20.2) | 10 607 (19.9) | 2270 (19.7) | |
| 4 | 48 108 (19.4) | 36 208 (19.7) | 9911 (18.6) | 1989 (17.3) | |
| 5 | 45 995 (18.5) | 35 238 (19.2) | 9035 (16.9) | 1722 (15.0) | |
| Residential status, rural, N (%) | 22 608 (9.1) | 17 159 (9.3) | 4574 (8.6) | 875 (7.6) | <0.001 |
| Long‐term care resident, N (%) | 2497 (1.0) | 1281 (0.0) | 942 (0.0) | 274 (0.0) | <0.001 |
| Estimated glomerular filtration rate, N (%) | |||||
| ≥60 mL/(min·1.73 m2) | 181 303 (73.0) | 142 447 (77.6) | 34 085 (63.8) | 4771 (41.5) | <0.001 |
| 45 to 59 mL/(min·1.73 m2) | 41 247 (16.6) | 28 423 (15.5) | 10 331 (19.3) | 2493 (21.7) | |
| 30 to 44 mL/(min·1.73 m2) | 19 099 (7.7) | 10 412 (5.7) | 6394 (12.0) | 2293 (19.9) | |
| <30 mL/(min·1.73 m2) | 6789 (2.7) | 2240 (1.2) | 2597 (4.9) | 1952 (17.0) | |
| Comorbidities | |||||
| Angina, N (%) | 2248 (0.9%) | 1471 (0.8) | 625 (1.2) | 152 (1.3) | <0.001 |
| Heart valve replacement, N (%) | 560 (0.2%) | 371 (0.2) | 160 (0.3) | 29 (0.3) | <0.001 |
| Hypertension, N (%) | 192 316 (77.4%) | 139 174 (75.8) | 43 279 (81.0) | 9863 (85.7) | <0.001 |
| Diabetes mellitus, N (%) | 128 212 (51.6%) | 88 678 (48.3) | 31 722 (59.4) | 7812 (67.9) | <0.001 |
| Dyslipidemia, N (%) | 6057 (2.4%) | 3892 (2.1) | 1664 (3.10) | 501 (4.4) | <0.001 |
| Atrial fibrillation/flutter, N (%) | 8023 (3.2) | 4480 (2.4) | 2821 (5.3) | 722 (6.3) | <0.001 |
| Stroke/transient ischemic attack, N (%) | 4402 (1.8) | 2727 (1.5) | 1304 (2.4) | 371 (3.2) | <0.001 |
| Venous thromboembolism, N (%) | 4180 (1.7) | 2899 (1.6) | 1031 (1.9) | 250 (2.2) | <0.001 |
| Health care utilization | |||||
| Hospitalizations, median (25th, 75th percentiles) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | <0.001 |
| Emergency department visits, median (25th, 75th percentiles) | 0 (0, 0) | 0 (0, 0) | 0 (0, 1) | 0 (0, 1) | <0.001 |
| Nephrologist visits, N (%) | |||||
| 0 | 231 969 (93.4) | 175 806 (95.8) | 47 747 (89.4) | 8416 (73.1) | <0.001 |
| 1 to 3 | 14 546 (5.9) | 7100 (3.9) | 4991 (9.3) | 2455 (21.3) | |
| >3 | 1923 (0.8) | 616 (0.3) | 669 (1.3) | 638 (5.5) | |
| Cardiologist visits, N (%) | |||||
| 0 | 216 181 (87.0) | 161 417 (88.0) | 45 265 (84.8) | 9499 (82.5) | <0.001 |
| 1 to 3 | 27 975 (11.3) | 19 452 (10.6) | 6885 (12.9) | 1638 (14.2) | |
| >3 | 4282 (1.7) | 2653 (1.4) | 1257 (2.4) | 372 (3.2) | |
| Adjusted diagnostic groups, N (%) | |||||
| 0 to 4 | 56 323 (22.7) | 42 509 (23.0) | 11 612 (22.0) | 2202 (19.0) | <0.001 |
| 5 to 9 | 122 712 (49.4) | 92 277 (50.0) | 25 188 (47.0) | 5247 (46.0) | |
| 10 to 14 | 60 043 (24.2) | 42 729 (23.0) | 14 003 (26) | 3311 (29.0) | |
| 15 to 19 | 9052 (3.6) | 5827 (3.0) | 2519 (5.0) | 706 (6.0) | |
| 20+ | 308 (0.1) | 180 (0.0) | 85 (0.0) | 43 (0.0) | |
| Resource utilization bands, N (%) | |||||
| 0 | 594 (0.2) | 442 (0.0) | 126 (0.0) | 26 (0.0) | <0.001 |
| 1 (low) | 1408 (0.6) | 1114 (1.0) | 253 (1.0) | 41 (0.0) | |
| 2 | 13 459 (5.4) | 10 254 (6.0) | 2723 (5.0) | 482 (4.0) | |
| 3 | 135 585 (54.6) | 103 872 (57.0) | 26 650 (50.0) | 5063 (44.0) | |
| 4 | 61 060 (24.6) | 44 308 (24.0) | 13 693 (26.0) | 3059 (27.0) | |
| 5 (high) | 36 332 (14.6) | 23 532 (13.0) | 9962 (19.0) | 2838 (25.0) | |
| Medications | |||||
| β‐Blockers, N (%) | 58 662 (23.6) | 39 297 (21.4) | 15 379 (28.8) | 3986 (34.6) | <0.001 |
| Antihypertensive agents, N (%) | 141 912 (57.1) | 98 128 (53.5) | 35 072 (65.7) | 8712 (75.7) | <0.001 |
| Statins, N (%) | 115 629 (46.5) | 83 626 (45.6) | 25 859 (48.4) | 6144 (53.4) | <0.001 |
| Antiplatelet agents, N (%) | 20 557 (8.3) | 13 386 (7.3) | 5692 (10.7) | 1479 (12.9) | <0.001 |
| Anticoagulants, N (%) | 14 195 (5.7) | 8302 (4.5) | 4756 (8.9) | 1137 (9.9) | <0.001 |
STEMI and NSTEMI Events in Follow‐Up
A total of 1436 (0.58%) STEMI and 4431 (1.78%) NSTEMI hospitalizations were observed over 1.1 million total person‐years of follow‐up. Table 2 and Figures 2 and 3 show the number of events and incidence rates per 100 000 person‐years stratified by ACR and eGFR risk categories. Stepwise increases in the incidence rates of STEMI and NSTEMI were observed with higher ACR and lower eGFR categories. The incidence rate of NSTEMI was higher compared to STEMI across all risk categories. Within ACR risk categories, the incidence rates of STEMI and NSTEMI were higher with lower eGFR categories, with the exception of those with an ACR <3 mg/mmol. Within eGFR risk categories, the incidence rates of STEMI and NSTEMI were higher with higher ACR categories, with the exception of those with an eGFR of 45 to 59 mL/(min·1.73 m2).
Table 2.
STEMI and NSTEMI Events and Incidence Rate Per 100 000 Person‐Years in 5‐Year Follow‐Up, Stratified by Albumin‐to‐Creatinine Ratio and eGFR Risk Categories
| eGFR (mL/[min·1.73 m2]) | Albumin‐to‐Creatinine Ratio (mg/mmol) | |||||
|---|---|---|---|---|---|---|
| <3 | 3 to 30 | >30 | ||||
| N (%) | IR (95% CI) | N (%) | IR (95% CI) | N (%) | IR (95% CI) | |
| STEMI | ||||||
| ≥60 | 637 (0.4) | 0.97 (0.89, 1.05) | 228 (0.7) | 1.50 (1.31, 1.70) | 45 (0.9) | 2.18 (1.59, 2.92) |
| 45 to 59 | 151 (0.5) | 1.16 (0.99, 1.37) | 89 (0.9) | 1.98 (1.59, 2.43) | 18 (0.7) | 1.71 (1.01, 2.70) |
| 30 to 44 | 86 (0.8) | 1.87 (1.50, 2.31) | 53 (0.8) | 2.00 (1.50, 2.62) | 38 (1.7) | 4.16 (2.94, 5.71) |
| <30 | 15 (0.7) | 1.65 (0.92, 2.72) | 28 (1.1) | 2.92 (1.94, 4.22) | 48 (2.5) | 7.00 (5.16, 9.28) |
| NSTEMI | ||||||
| ≥60 | 1534 (1.1) | 2.33 (2.21, 2.45) | 760 (2.2) | 4.99 (4.64, 5.36) | 151 (3.2) | 7.34 (6.21, 8.60) |
| 45 to 59 | 518 (1.8) | 3.99 (3.66, 4.35) | 296 (2.9) | 6.58 (5.85, 7.37) | 119 (4.8) | 11.33 (9.38, 13.56) |
| 30 to 44 | 305 (2.9) | 6.65 (5.92, 7.44) | 221 (3.5) | 8.38 (7.31, 9.56) | 131 (5.7) | 14.37 (12.01, 17.05) |
| <30 | 93 (4.2) | 10.27 (8.29, 12.58) | 150 (5.8) | 15.74 (13.32, 18.47) | 153 (7.8) | 22.47 (19.05, 26.33) |
CI indicates confidence interval; eGFR, estimated glomerular filtration rate; IR, incidence rate; NSTEMI, non–ST‐segment–elevation myocardial infarction; STEMI, ST‐segment–elevation myocardial infarction.
Figure 2.

Incidence rate per 100 000 person‐years of STEMI by levels of eGFR and ACR. ACR indicates albumin‐to‐creatinine ratio; eGFR, estimated glomerular filtration rate; STEMI, ST‐segment–elevation myocardial infarction.
Figure 3.

Incidence rate per 100 000 person‐years of non–ST‐elevation myocardial infarction by levels of estimated glomerular filtration rate and albumin‐to‐creatinine ratio. eGFR indicates estimated glomerular filtration rate; NSTEMI, non–ST‐segment–elevation myocardial infarction.
Compared with individuals with an ACR <3 mg/mmol and an eGFR ≥60 mL/(min·1.73 m2), there was an overall trend toward higher relative risks of STEMI and NSTEMI with a higher ACR and a lower eGFR (Table 3). Measures of kidney function were significant effect modifiers for both STEMI and NSTEMI (STEMI eGFR×ACR interaction P value 0.01, NSTEMI eGFR×ACR interaction P<0.0001). For individuals with an ACR >30 mg/mmol with an eGFR ≥60 mL/(min·1.73 m2), the adjusted risks of STEMI and NSTEMI were 2‐ and 2.5‐fold higher, respectively, than those in individuals with a low ACR (<3 mg/mmol) with a normal eGFR (STEMI sHR 1.96, 95% confidence interval 1.45‐2.66; NSTEMI sHR 2.46, 95% confidence interval 2.08‐2.91). Individuals with the lowest eGFR (<30 mL/[min·1.73 m2]) and the lowest ACR (<3 mg/mmol) had similar STEMI risks to those with a normal eGFR and the lowest ACR. However, individuals with the lowest eGFR and the lowest ACR had a nearly 4‐fold higher risk of NSTEMI compared with those having a normal eGFR and lowest ACR. For the combination of lowest eGFR (<30 mL/[min·1.73 m2]) and highest ACR (>30 mg/mmol), the adjusted risks of STEMI and NSTEMI were 4‐fold higher than those in people with an ACR <3 mg/mmol and eGFR <30 mL/(min·1.73 m2) (STEMI sHR 4.53, 95% confidence interval 3.30‐6.21; NSTEMI sHR 4.42, 95% confidence interval 3.67‐5.32).
Table 3.
Adjusted Hazard Ratios of STEMI and NSTEMI Stratified by Albumin‐to‐Creatinine Ratio and eGFR Risk Categories
| eGFR (mL/[min·1.73 m2]) | Albumin‐to‐Creatinine Ratio (mg/mmol) | ||
|---|---|---|---|
| <3 | 3 to 30 | >30 | |
| HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| STEMI | |||
| ≥60 | Referent | 1.40 (1.20‐1.63) | 1.96 (1.45‐2.66) |
| 45 to 59 | 1.07 (0.89‐1.28) | 1.57 (1.24‐1.97) | 1.37 (0.85‐2.19) |
| 30 to 44 | 1.55 (1.22‐1.97) | 1.43 (1.07‐1.92) | 2.99 (2.13‐4.19) |
| <30 | 1.17 (0.69‐1.98) | 1.88 (1.26‐2.79) | 4.53 (3.30‐6.21) |
| NSTEMI | |||
| ≥60 | Referent | 1.78 (1.63‐1.94) | 2.46 (2.08‐2.91) |
| 45 to 59 | 1.33 (1.20‐1.48) | 1.80 (1.58‐2.04) | 3.12 (2.58‐3.78) |
| 30 to 44 | 1.83 (1.61‐2.09) | 1.93 (1.66‐2.24) | 3.40 (2.82‐4.10) |
| <30 | 2.12 (1.78‐2.76) | 3.09 (2.57‐3.70) | 4.42 (3.67‐5.32) |
Models adjusted for age, sex, income quintile, residential status, long‐term care residence, year of index, hypertension, dyslipidemia, diabetes mellitus, obesity, angina, heart valve replacement, atrial fibrillation or flutter, stroke or transient ischemic attack, venous thromboembolism, hospitalizations, emergency department visits, nephrology visits, cardiology visits, adjusted clinical groups and resource utilization bands, and β‐blocker, antihypertensive, statin, antiplatelet, and oral anticoagulant prescription, as defined in baseline characteristics. STEMI: eGFR×ACR interaction P=0.01. NSTEMI: eGFR×ACR interaction P<0.0001. ACR indicates albumin‐to‐creatinine ratio; CI, confidence interval; eGFR, estimated glomerular filtration rate; HR, hazard ratio; NSTEMI, non‐ST‐segment‐elevation myocardial infarction; STEMI, STEMI, ST‐segment‐elevation myocardial infarction.
Discussion
In this large population‐based study, we found that CKD was independently associated with an increased risk of both STEMI and NSTEMI. An isolated elevation in albuminuria significantly increased the risk of both STEMI and NSTEMI, whereas an isolated decrease in eGFR only increased the risk of NSTEMI. The combination of low kidney function and elevated albuminuria was associated with a greater than 4‐fold higher risk of both MI types. Elevations in albuminuria demonstrated a more consistent higher risk of STEMI and may be a valuable and readily available test to improve risk prediction.
Our findings focused on individuals over the age of 66, a cohort traditionally defined as the age of retirement in the US Social Security Act.20 Our study cohort is highly relevant as the American population is aging, with the proportion of people over the age of 65 predicted to increase from 12% in the year 2000 to 20% by the year 2030.21 Furthermore, based on Medicare claims data, the prevalence of CKD is high among those of advanced age compared with younger individuals at 10% and 1.5%, respectively.21, 22 This greater burden of CKD among those with advanced age coincides with a high prevalence of traditional risk factors such as diabetes mellitus and hypertension.
Previous studies have clearly demonstrated a higher risk of MI and cardiovascular mortality with low eGFR and/or albuminuria.7, 8, 23, 24, 25 Hallan et al, examining data from the HUNT II Norwegian health study, found a 6.7‐fold higher adjusted cardiovascular mortality risk for individuals with an eGFR <45 mL/(min·1.73 m2) and high ACR compared to an eGFR >75 mL/(min·1.73 m2) and a normal ACR.7 In 920 985 individuals in Alberta, Canada, Hemmelgarn et al reported a stepwise increase in the adjusted MI rate with an increase in ACR, a decrease in eGFR, or a combination of both.8, 26 However, data on MI type, specifically the relationship between STEMI and kidney disease, are limited. A report by the National Cardiovascular Data ACTION Registry identifies MI type by eGFR categories but lacks information on albuminuria.9 Akerblom et al reported an association of an alternative kidney filtration marker (cystatin C) and acute coronary syndrome that did not differ by MI type.27 However, the study lacked albuminuria data and examined patients with a relatively high median eGFR. Our study clearly identifies the differing risk profile in MI type by a higher ACR, a lower eGFR, and their combination. The elevated risk of STEMI was evident and related to higher albuminuria excretion with no increase in risk with an isolated lower eGFR level.
Mechanistically, the presence of albuminuria or a low eGFR seem to exhibit different effects on vasculature. Coronary artery calcification has been associated with adverse cardiovascular events, and studies have shown that lower levels of eGFR are associated with a greater propensity for vascular calcification.28, 29 The presence of nontraditional risk factors in CKD such as abnormal mineral metabolism, predominantly hyperphosphatemia and hypercalcemia, likely facilitates progression of vascular calcification and subsequent NSTEMI.6, 30 This was illustrated by the nearly 4‐fold higher risk of NSTEMI with low levels of eGFR in the absence of albuminuria observed in our study. Conversely, albuminuria has been demonstrated to be an independent risk factor for arterial thromboembolism.31, 32 In 1989, the Steno Hypothesis proposed that proteinuria reflects a generalized dysfunction in vascular endothelium.33 Since then, studies have shown that factors associating proteinuria with increased cardiovascular risk include vascular endothelial growth factor, inflammation, and thrombotic factors. The pathogenesis of proteinuria related to vascular endothelial growth factor is not clear, but endothelial dysfunction is a potential cause because it is important in the maintenance of endothelial function and endothelial repair after injury.34, 35 Yilmaz et al showed that proteinuria is associated with asymmetric dimethylarginine, an inflammatory biomarker that inhibits nitric oxide production, which results in endothelial dysfunction and atherosclerosis.36 High‐sensitivity C‐reactive protein is associated with both a higher global cardiometabolic risk37 and increasing levels of proteinuria.38, 39 Finally, von Willebrand factor, soluble vascular cell adhesion molecule, fibrinogen, and tissue plasminogen activator are elevated with higher levels of urinary albumin excretion.40 Last, elevations in albuminuria may arise from 2 distinct but interrelated processes because it is a marker of endothelial dysfunction and/or elevated blood pressure.
Our findings from a large population‐level cohort with access to universal healthcare are generalizable and build on previous findings. Using well‐validated definitions we were able to capture a large number of STEMI and NSTEMI events, which allowed us to determine the relative risks across multiple eGFR/ACR combinations. Despite these strengths, our study does have limitations. We defined CKD by single outpatient measures of eGFR and ACR, which could lead to a potential misclassification, of acute kidney injury as CKD. However, previous validation studies demonstrate an improved degree of accuracy when utilizing outpatient rather than inpatient serum creatinine measures.13 We included a large number of clinically important covariates in our models, including medications and healthcare utilization; however, some important potential confounders were unavailable. Certain antiplatelet agents (acetylsalicylic acid) are available without a prescription in Ontario; thus, we may have underestimated its use in our population. However, we adjusted for proxies in our models such as angina and stroke that often are associated with antiplatelet use. We lacked specific information on blood pressure and again used proxies of a previous diagnosis of hypertension or prescription of an antihypertensive medication in our analysis. There is a potential for misclassification of acute coronary syndrome type, as the outcome definition for STEMI and NSTEMI we used reported misclassification of 7.7% of patients with STEMI as NSTEMI in the original validation study. Albuminuria measures are more commonly performed in patients with diabetes mellitus, which has likely resulted in these patients being oversampled in this study.
In this population‐level study, we have shown specifically that isolated albuminuria confers a higher risk of both types of MI (STEMI and NSTEMI), regardless of eGFR, whereas isolated declines in eGFR are associated with a higher risk of NSTEMI. Taken together, our results suggest that albuminuria and eGFR may be helpful in predicting the risk of MI type in individuals, and this may aid clinicians in targeting appropriate therapies and preventative measures.
Sources of Funding
This study was supported by the Institute for Clinical Evaluative Sciences (ICES) Western and Ottawa Site. ICES is funded by an annual grant from the Ontario Ministry of Health and Long‐Term Care. Core funding for ICES Western is provided by the Academic Medical Organization of Southwestern Ontario, the Schulich School of Medicine and Dentistry, Western University, and the Lawson Health Research Institute. The research was conducted by members of the ICES Kidney, Dialysis, and Transplantation team, at the ICES Ottawa and Western facilities, which are supported by a grant from the Canadian Institutes of Health Research. The opinions, results and conclusions are those of the authors and are independent of the funding sources. No endorsement by any of the organizations named above is intended or should be inferred.
Disclosures
None.
Supporting information
Table S1. Checklist of Recommendations for Reporting of Observational Studies Using the RECORD Statement
Acknowledgments
Parts of this material are based on data and/or information compiled and provided by Canadian Institute for Health Information (CIHI). However, the analyses, conclusions, opinions, and statements expressed in the material are those of the authors and not necessarily those of CIHI. We thank Gamma‐Dynacare (Brampton, ON, Canada) for laboratory data and IMS Brogan Inc (Kanata, ON, Canada) for use of their Drug Information Database.
(J Am Heart Assoc. 2018;7:e009995 DOI: 10.1161/JAHA.118.009995.)
References
- 1. Arora P, Vasa P, Brenner D, Iglar K, McFarlane P, Morrison H, Badawi A. Prevalence estimates of chronic kidney disease in Canada: results of a nationally representative survey. Can Med Assoc J. 2013;185:E417–E423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2003;41:1–12. [DOI] [PubMed] [Google Scholar]
- 3. Jha V, Garcia‐Garcia G, Iseki K, Li Z, Naicker S, Plattner B, Saran R, Wang AY‐M, Yang C‐W. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;382:260–272. [DOI] [PubMed] [Google Scholar]
- 4. Tonelli M, Wiebe N, Culleton B, House A, Rabbat C, Fok M, McAlister F, Garg AX. Chronic kidney disease and mortality risk: a systematic review. J Am Soc Nephrol. 2006;17:2034–2047. [DOI] [PubMed] [Google Scholar]
- 5. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu C‐Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. [DOI] [PubMed] [Google Scholar]
- 6. Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW; American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention . Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation. 2003;108:2154–2169. [DOI] [PubMed] [Google Scholar]
- 7. Hallan S, Astor B, Romundstad S, Aasarød K, Kvenild K, Coresh J. Association of kidney function and albuminuria with cardiovascular mortality in older vs younger individuals: the HUNT II Study. Arch Intern Med. 2007;167:2490–2496. [DOI] [PubMed] [Google Scholar]
- 8. Hemmelgarn BR, Manns BJ, Lloyd A, James MT, Klarenbach S, Quinn RR, Wiebe N, Tonelli M; Alberta Kidney Disease Network . Relation between kidney function, proteinuria, and adverse outcomes. JAMA. 2010;303:423–429. [DOI] [PubMed] [Google Scholar]
- 9. Fox CS, Muntner P, Chen AY, Alexander KP, Roe MT, Cannon CP, Saucedo JF, Kontos MC, Wiviott SD. Use of evidence‐based therapies in and short‐term outcomes of STEMI and NSTEMI in patients with chronic kidney disease: a report from the National Cardiovascular Data ACTION Registry. Circulation. 2010;121:357–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jha P, Deboer D, Sykora K, Naylor CD. Characteristics and mortality outcomes of thrombolysis trial participants and nonparticipants: a population‐based comparison. J Am Coll Cardiol. 1996;27:1335–1342. [DOI] [PubMed] [Google Scholar]
- 11. Benchimol EI, Smeeth L, Guttmann A, Harron K, Moher D, Petersen I, Sørensen HT, von Elm E, Langan SM; RECORD Working Committee . The reporting of studies conducted using observational toutinely‐collected health data (RECORD) statement. PLoS Med. 2015;12:e1001885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Levey AS, Stevens LA, Schmid CH, Zhang Y, Castro AF III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Garg AX, Mamdani M, Juurlink DN, van Walraven C; Network of Eastern Ontario Medical Laboratories . Identifying individuals with a reduced GFR using ambulatory laboratory database surveillance. J Am Soc Nephrol. 2005;16:1433–1439. [DOI] [PubMed] [Google Scholar]
- 14. KDIGO CKD Kidney Working Group . Clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150. [Google Scholar]
- 15. Weiner JP, ed. The Johns Hopkins University Bloomberg School of Public Health HSRDC: the Johns Hopkins ACG® Case‐Mix System Version 10.0 release notes. Baltimore, MD: The Johns Hopkins University; 2011. [Google Scholar]
- 16. Levy AR, O'Brien BJ, Sellors C, Grootendorst P, Willison D. Coding accuracy of administrative drug claims in the Ontario Drug Benefit database. Can J Clin Pharmacol. 2003;10:67–71. [PubMed] [Google Scholar]
- 17. Patel AB, Quan H, Welsh RC, Deckert‐Sookram J, Tymchak W, Sookram S, Surdhar I, Kaul P. Validity and utility of ICD‐10 administrative health data for identifying ST‐ and non‐ST‐elevation myocardial infarction based on physician chart review. CMAJ Open. 2015;3:E413–E418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hsu JY, Roy JA, Xie D, Yang W, Shou H, Anderson AH, Landis JR, Jepson C, Wolf M, Isakova T, Rahman M, Feldman HI; Chronic Renal Insufficiency Cohort (CRIC) Study Investigators . Statistical methods for cohort studies of CKD: survival analysis in the setting of competing risks. Clin J Am Soc Nephrol. 2017;12:1181–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Harrell FE. Multivariable modeling strategies In: Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis. New York, NY: Springer International Publishing; 2015:63–102. [Google Scholar]
- 20. Waller CE. The Social Security Act in its relation to public health. Am J Public Health Nations Health. 1935;25:1186–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Centers for Disease Control and Prevention: trends in aging—United States and worldwide. MMWR Morb Mortal Wkly Rep. 2003;52:101–104, 106. [PubMed] [Google Scholar]
- 22. USRDS: the United States renal data system. Am J Kidney Dis. 2003;42(6 suppl 5):1–230. [PubMed] [Google Scholar]
- 23. Mann JE, Gerstein HC, Pogue J, Bosch J, Yusuf S. Renal insufficiency as a predictor of cardiovascular outcomes and the impact of ramipril: the HOPE randomized trial. Ann Intern Med. 2001;134:629–636. [DOI] [PubMed] [Google Scholar]
- 24. Tonelli M, Jose P, Curhan G, Sacks F, Braunwald E, Pfeffer M. Proteinuria, impaired kidney function, and adverse outcomes in people with coronary disease: analysis of a previously conducted randomised trial. BMJ. 2006;332:1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Levey AS, de Jong PE, Coresh J, Nahas MEl, Astor BC, Matsushita K, Gansevoort RT, Kasiske BL, Eckardt K‐U. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int. 2011;80:17–28. [DOI] [PubMed] [Google Scholar]
- 26. Bello AK, Hemmelgarn B, Lloyd A, James MT, Manns BJ, Klarenbach S, Tonelli M; Alberta Kidney Disease Network . Associations among estimated glomerular filtration rate, proteinuria, and adverse cardiovascular outcomes. Clin J Am Soc Nephrol. 2011;6:1418–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Akerblom Å, Wallentin L, Siegbahn A, Becker RC, Budaj A, Buck K, Giannitsis E, Horrow J, Husted S, Katus HA, Steg PG, Storey RF, Åsenblad N, James SK. Cystatin C and estimated glomerular filtration rate as predictors for adverse outcome in patients with ST‐elevation and non–ST‐elevation acute coronary syndromes: results from the platelet inhibition and patient outcomes study. Clin Chem. 2012;58:190. [DOI] [PubMed] [Google Scholar]
- 28. Bundy JD, Chen J, Yang W, Budoff M, Go AS, Grunwald JE, Kallem RR, Post WS, Reilly MP, Ricardo AC, Rosas SE, Zhang X, He J; CRIC Study Investigators . Risk factors for progression of coronary artery calcification in patients with chronic kidney disease: the CRIC study. Atherosclerosis. 2018;271:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Anderson S, Halter JB, Hazzard WR, Himmelfarb J, Horne FM, Kaysen GA, Kusek JW, Nayfield SG, Schmader K, Tian Y, Ashworth JR, Clayton CP, Parker RP, Tarver ED, Woolard NF, High KP; Workshop Participants . Prediction, progression, and outcomes of chronic kidney disease in older adults. J Am Soc Nephrol. 2009;20:1199–1209. [DOI] [PubMed] [Google Scholar]
- 30. Tuttle KR, Short RA. Longitudinal relationships among coronary artery calcification, serum phosphorus, and kidney function. Clin J Am Soc Nephrol. 2009;4:1968–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Go AS, Fang MC, Udaltsova N, Chang Y, Pomernacki NK, Borowsky L, Singer DE; ATRIA Study Investigators . Impact of proteinuria and glomerular filtration rate on risk of thromboembolism in atrial fibrillation: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. Circulation. 2009;119:1363–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Piccini JP, Stevens SR, Chang Y, Singer DE, Lokhnygina Y, Go AS, Patel MR, Mahaffey KW, Halperin JL, Breithardt G, Hankey GJ, Hacke W, Becker RC, Nessel CC, Fox KA, Califf RM; ROCKET AF Steering Committee and Investigators . Renal dysfunction as a predictor of stroke and systemic embolism in patients with nonvalvular atrial fibrillation: validation of the R2CHADS2 index in the ROCKET AF (Rivaroxaban Once‐daily, oral, direct factor Xa inhibition Compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation) and ATRIA (AnTicoagulation and Risk factors in Atrial fibrillation) study cohorts. Circulation. 2013;127:224–232. [DOI] [PubMed] [Google Scholar]
- 33. Deckert T, Feldt‐Rasmussen B, Borch‐Johnsen K, Jensen T, Kofoed‐Enevoldsen A. Albuminuria reflects widespread vascular damage. The Steno hypothesis. Diabetologia. 1989;32:219–226. [DOI] [PubMed] [Google Scholar]
- 34. Kim NH, Oh JH, Seo JA, Lee KW, Kim SG, Choi KM, Baik SH, Choi DS, Kang YS, Han SY, Han KH, Ji YH, Cha DR. Vascular endothelial growth factor (VEGF) and soluble VEGF receptor FLT‐1 in diabetic nephropathy. Kidney Int. 2005;67:167–177. [DOI] [PubMed] [Google Scholar]
- 35. Miyamoto K, Kitamoto Y, Tokunaga H, Takeya M, Ezaki T, Imamura T, Tomita K. Protective effect of vascular endothelial growth factor/vascular permeability factor 165 and 121 on glomerular endothelial cell injury in the rat. Lab Invest. 2004;84:1126–1136. [DOI] [PubMed] [Google Scholar]
- 36. Yilmaz MI, Sonmez A, Saglam M, Qureshi AR, Carrero JJ, Caglar K, Eyileten T, Cakir E, Oguz Y, Vural A, Yenicesu M, Lindholm B, Stenvinkel P, Axelsson J. ADMA levels correlate with proteinuria, secondary amyloidosis, and endothelial dysfunction. J Am Soc Nephrol. 2008;19:388–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fort J. Chronic renal failure: a cardiovascular risk factor. Kidney Int Suppl. 2005;S25–S29. [DOI] [PubMed] [Google Scholar]
- 38. Agarwal R, Andersen MJ. Correlates of systolic hypertension in patients with chronic kidney disease. Hypertension. 2005;46:514–520. [DOI] [PubMed] [Google Scholar]
- 39. Caglar K, Yilmaz MI, Sonmez A, Cakir E, Kaya A, Acikel C, Eyileten T, Yenicesu M, Oguz Y, Bilgi C, Oktenli C, Vural A, Zoccali C. ADMA, proteinuria, and insulin resistance in non‐diabetic stage I chronic kidney disease. Kidney Int. 2006;70:781–787. [DOI] [PubMed] [Google Scholar]
- 40. Stehouwer CD, Gall MA, Twisk JW, Knudsen E, Emeis JJ, Parving HH. Increased urinary albumin excretion, endothelial dysfunction, and chronic low‐grade inflammation in type 2 diabetes: progressive, interrelated, and independently associated with risk of death. Diabetes. 2002;51:1157–1165. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Checklist of Recommendations for Reporting of Observational Studies Using the RECORD Statement


