Extended Data Fig. 1 |. The CYCT1 HRD-dependent hyperphosphorylation of Pol II CTD52 by affinity-purified CDK9– CYCT1–Flag dimer can also be detected with anti-phospho-Ser2 antibody.
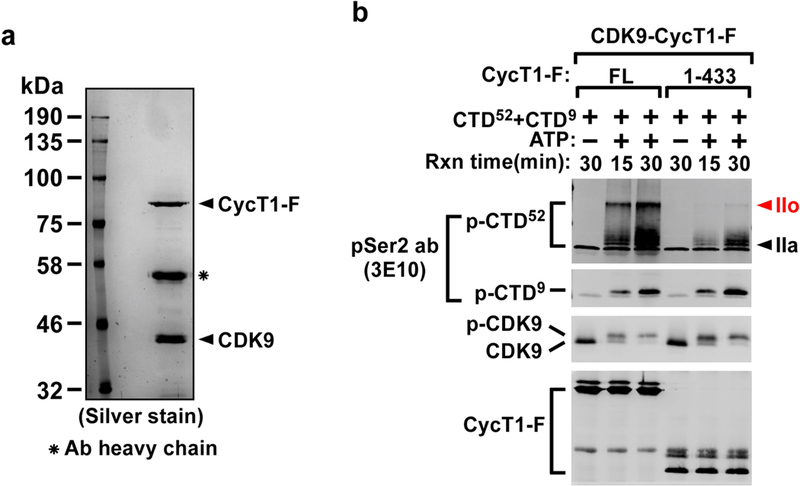
a, Examination of the affinity-purified CDK9–CYCT1–Flag dimer containing wild-type CYCT1–Flag by SDS–PAGE and silver staining. The anti-Flag affinity-purification was performed under high salt plus detergent (1 M KCl + 1% NP-40) conditions to strip away all the P-TEFb-associated factors but keep the CDK9–CYCT1 interaction intact. b, Affinity-purified CDK9–CYCT1–Flag heterodimers containing the indicated CYCT1–Flag proteins were tested in kinase reactions containing a mixture of GST-fused CTD52 and CTD9 as the substrates. The phosphorylated p-CTD52 and p-CTD 9 were detected by western blotting with the anti-phospho-Ser2 antibody 3E10. Although a very similar pattern of CTD phosphorylation was detected with both anti-phospho-Ser2 and anti-phospho-Ser5 antibodies, the unphosphorylated CTD present in the ATP(–) lanes was only detected by the former antibody, making the phospho-Ser5 antibody a preferred choice for detecting CTD phosphorylation in these kinase reactions.
