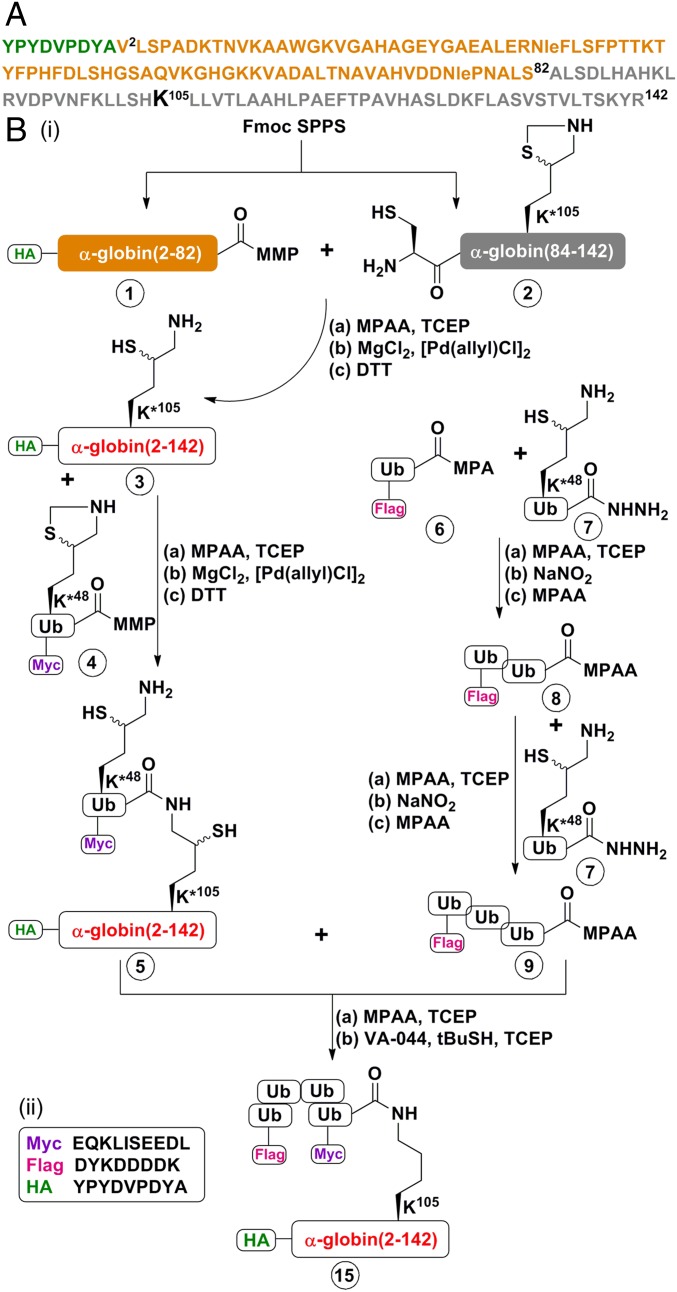
Fig. 1.
Representative scheme of the strategy used for chemical synthesis of differentially tagged tetra-Ub HA-α-globin. A detailed description of the complete synthesis of the building blocks and intermediates for the generation of HA-α-globin constructs is provided in SI Appendix, Figs. S1–S23. (A) Sequence of human HA-α-globin. The HA tag sequence is in green. The fragment 1 sequence (residues 2–82) is in light brown, and the fragment 2 sequence (residues Cys83-84–142) is in gray. Note that Ala83 in the native α-globin was substituted with Cys to enable chemical ligation and later desulfurized back to Ala. (B, i) Chemical synthesis steps of tetra-Ub HA-α-globin (construct 15). HA-α-globin K*105 (intermediate 3; K*105 denotes the presence of δ-mercaptolysine at this position) was generated from the ligation of fragments 1 and 2 of α-globin. Note that native α-globin contains a Cys residue at position 105. The ubiquitination sites of globins have not been identified; a previous study reported successful ubiquitination at position 105, generating a thiolester bond on the native Cys residue (15). In the present study, to generate an isopeptide bond, the native Cys residue was replaced with δ-mercaptolysine. Intermediate 3 was next ligated with (intermediate 4Myc-Ub(K*48)-MMP; K*48 denotes the presence of δ-mercaptolysine at this position) to obtain intermediate 5, which contains the whole HA-α-globin to which one Myc-Ub moiety is bound. In parallel, Flag-tri-Ub-MPAA (intermediate 9) was assembled from Ub monomers. Intermediates 5 and 9 were then ligated and desulfurized to obtain the final Flag-Ub-Ub-Ub-Myc-Ub-(tetra-Ub)-HA-α-globin (construct 15). (a), (b), and (c) denote the reagents used for each reaction. Details of the materials used and the synthetic steps are provided in SI Appendix, Materials and Methods). (B, ii) Sequences of Myc, Flag, and HA tags.