Fig. 2.
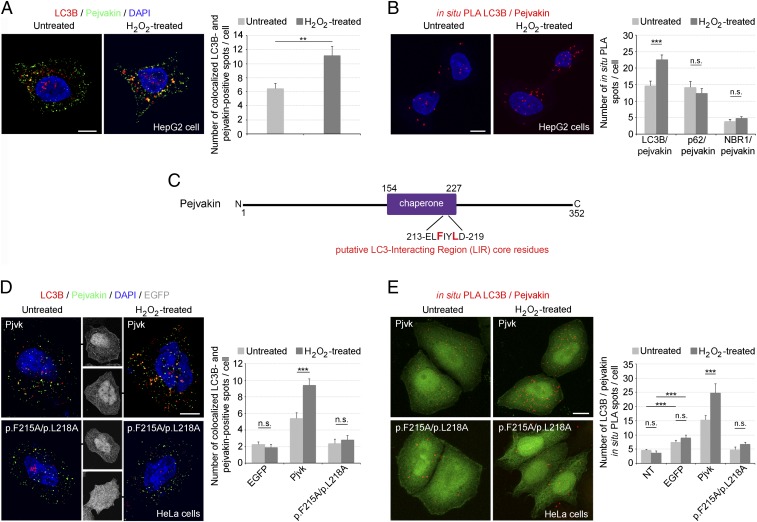
Pejvakin recruits LC3B directly via its LIR, promoting autophagy in response to oxidative stress. (A) Coimmunolabeling of pejvakin and LC3B in untreated and H2O2-treated HepG2 cells. The number of LC3B-positive spots colocalizing with PMP70 labeling increased after oxidative stress (bar charts; n = 20 cells per condition). (B) Interactions between LC3B and pejvakin, detected as in situ PLA spots, in untreated and H2O2-treated HepG2 cells. The bar charts show an increase in the number of LC3B/pejvakin PLA spots after oxidative stress but no change in the number of p62/pejvakin or NBR1/pejvakin PLA spots (n = 20 cells per condition). (C) Schematic representation of the putative LIR motif (core residues in red) in the predicted pejvakin chaperone domain. (D and E) Interactions between pejvakin and LC3B in untransfected HeLa cells (NT) and transfected HeLa cells producing EGFP alone, EGFP and wild-type pejvakin (Pjvk), or EGFP and pejvakin with mutated core residues. p.F215A and p.L218A were detected by double-immunolabeling (D) and in situ PLA (E) for pejvakin and LC3B before and after oxidative stress. The bar charts in D and E show that H2O2 treatment increases LC3B recruitment to pejvakin in transfected HeLa cells producing EGFP and wild-type pejvakin but not in cells producing mutated pejvakin or untransfected cells (n = 20 cells per condition). Data are mean ± SEM. **P < 0.01; ***P < 0.001; n.s., not significant, unpaired Student’s t test. (Scale bars: 5 μm.)