Fig. 5.
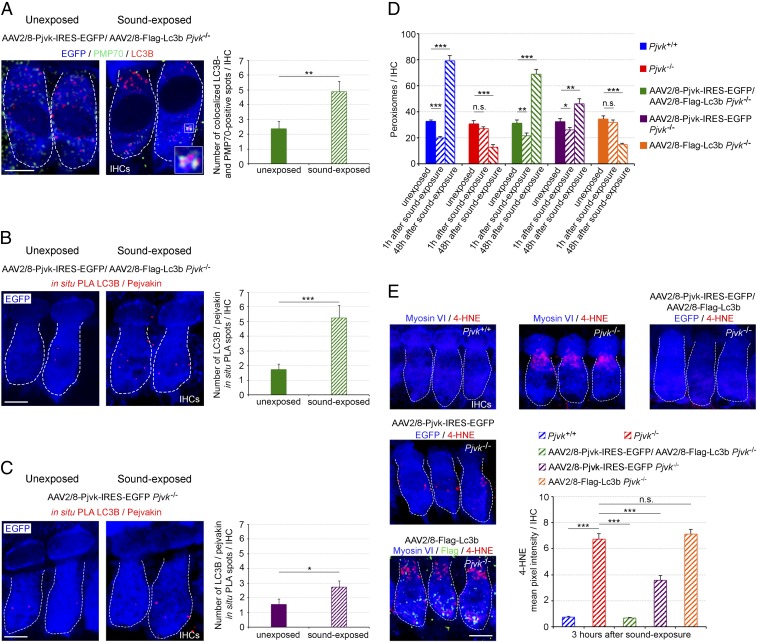
Transfer of Pjvk and Lc3b into Pjvk−/− IHCs restores pexophagy and abolishes lipid peroxidation after sound exposure. (A) The number of LC3B-positive spots colocalizing with PMP70 labeling was higher at 1 h after sound exposure (5–40 kHz, 105 dB SPL, 1h) in Pjvk−/− IHCs cotransduced with AAV2/8-Pjvk-IRES-EGFP and AAV2/8-Flag-Lc3b. (Inset) LC3B recruitment to peroxisomes. (B) The number of LC3B/pejvakin in situ PLA spots was increased at 1 h after sound exposure in Pjvk−/− IHCs cotransduced with AAV2/8-Pjvk-IRES-EGFP and AAV2/8-Flag-Lc3b. (C) The number of LC3B/pejvakin in situ PLA spots had increased slightly at 1 h after sound exposure in Pjvk−/− IHCs transduced with AAV2/8-Pjvk-IRES-EGFP. The bar charts in A–C quantify LC3B-positive spots colocalizing with PMP70 labeling (A) and LC3B/pejvakin in situ PLA spots (B and C) in treated IHCs before and after sound exposure (n = 40 IHCs; four mice per condition). (D) Peroxisome degradation (at 1 h after sound exposure) and proliferation (at 48 h after sound exposure) were fully restored in Pjvk−/− IHCs cotransduced with AAV2/8-Pjvk-IRES-EGFP and AAV2/8-Flag-Lc3b. AAV2/8-Pjvk-IRES-EGFP transduction of Pjvk−/− IHCs partially rescued early pexophagy and proliferation, whereas the transduction of Pjvk−/− IHCs with AAV2/8-Flag-Lc3b had no effect (n = 40 IHCs from four mice). (E) Lipid peroxidation levels, as assessed by 4-HNE immunolabeling, were higher in Pjvk−/− IHCs than in Pjvk+/+ IHCs at 3 h after sound exposure. Lipid peroxidation was abolished in Pjvk−/− IHCs cotransduced with AAV2/8-Pjvk-IRES-EGFP and AAV2/8-Flag-Lc3b but was significantly increased in both Pjvk−/− IHCs and AAV2/8-Flag-Lc3b Pjvk−/− IHCs. 4-HNE immunoreactivity was weaker in AAV2/8-Pjvk-IRES-EGFP Pjvk−/− IHCs than in Pjvk−/− IHCs (n = 50 cells; four mice per condition). Data are mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001; n.s., not significant, unpaired Student’s t test. (Scale bars: 5 μm.)