Figure 3.
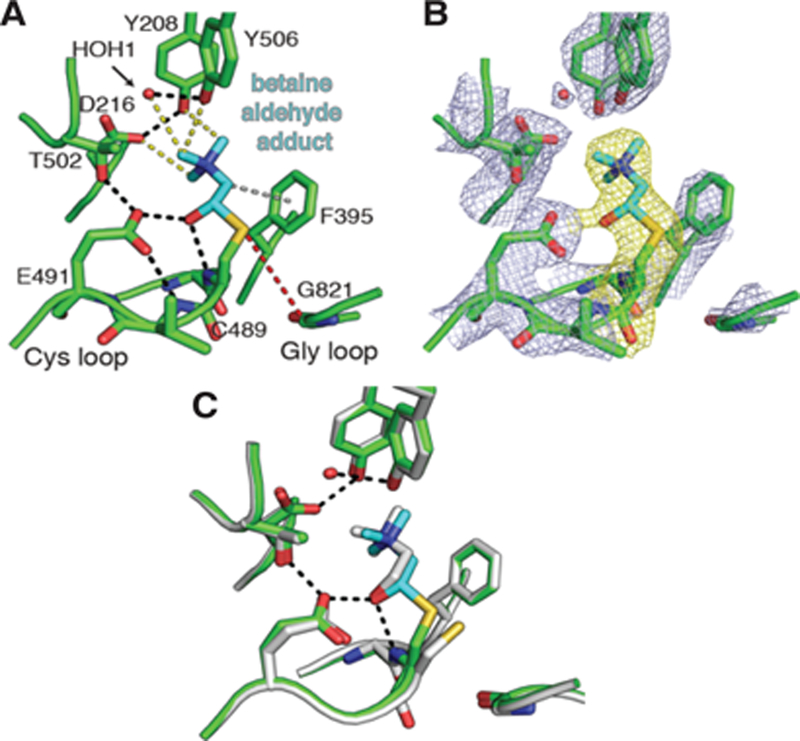
Betaine aldehyde binding to CutC appears to involve a covalent, thiohemiacetal linkage. (A) Crystal structure of unactivated CutC soaked with betaine aldehyde (10 mM) reveals interactions between CutC active site residues and bound inhibitor (cyan). Shown are traditional hydrogen bonds (2.5–3.2 Å, black dashes), CH—O hydrogen bonds (3.2–3.7 Å, yellow dashes), and a cation-π-like interaction (gray dashes). (B) Composite omit 2Fo-Fc density (light blue) contoured at 1.8σ. Density surrounding betaine aldehydemodified Cys493 is highlighted (yellow). (C) Comparison of inhibitor-bound (green/cyan) and choline-bound (grey) CutC structures. Hydrogen bonding interactions with the Cys loop (black dashes) are shown.
