Abstract
Every year, neurodegenerative disorders take more than 5,000 lives in the US alone. Cures have not yet been found for many of the multitude of neuropathies. The majority of amyotrophic lateral sclerosis (ALS), frontotemporal dementia (FTD) and Parkinson’s disease (PD) cases have no known genetic basis. Thus, it is evident that contemporary genetic approaches have failed to explain the etiology or etiologies of ALS/FTD and PD. Recent investigations have explored the potential role of epigenetic mechanisms in disease development. Epigenetics comprises heritable changes in gene utilization that are not derived from changes in the genome. A main epigenetic mechanism involves the post-translational modification of histones. Increased knowledge of the epigenomic landscape of neurodegenerative diseases would not only further our understanding of the disease pathologies, but also lead to the development of treatments able to halt their progress. Here, we review recent advances on the association of histone post-translational modifications with ALS, FTD, PD and several ataxias.
Keywords: amyotrophic lateral sclerosis, frontotemporal dementia, Parkinson’s disease, spinocerebellar ataxia, epigenetics, post translational modifications, histones, neurodegenerative disease, neurodegeneration, Friedreich’s ataxia
1. INTRODUCTION
As our population ages, neurodegenerative diseases have become a pressing problem.[1], [2] These disorders are incurable, relentless and often fatal shortly after diagnosis.[2]–[4] While these ailments vary in their specific pathophysiology and clinical presentation, they share several puzzling features. For instance, despite intense genetic analysis of large patient populations, a significant proportion of neurodegenerative disease cases have no known genetic basis.[5] Furthermore, several of these disorders show proteinaceous inclusions comprised of misfolded proteins.[6] Throughout the years, various medications and therapies have been considered for these diseases, many resulting in less than satisfactory results.[7] Hence, the need for novel treatments able to ameliorate symptoms and stop disease progress is at an all-time high.
1.1. Amyotrophic Lateral Sclerosis and Frontotemporal Dementia
Amyotrophic lateral sclerosis (ALS), also known as Lou Gehrig’s disease, is a neurological disorder characterized by the loss of function of motor neurons and resulting muscle weakness.[8] In 2016, approximately 15,000 people in the United States were suffering from ALS. Furthermore, nearly 5,000 new cases surface every year.[8] Many ALS patients ultimately perish from respiratory failure due to paralysis of muscles necessary for breathing, usually between two to four years after diagnosis.[9] At present, Rilutek (riluzole) and Radicava (edaravone) are the only FDA approved drugs for ALS treatment.[10], [11] Riluzole’s mechanism of action involves impacting glutamate release leading to protection against glutamate-induced slowing of neurofilament axonal transport,[12] while the mechanism of action of edaravone is currently unknown.[13] While succeeding in reducing ALS symptoms and modestly expanding the lifespan of ALS patients, these medications are not able to stop the progress of the disease. A highly effective cure for both ALS and FTD is yet to be discovered.
Most ALS cases arise in individuals with no family history of the disease; thus, they are termed sporadic. Only 5–10% of all ALS cases are linked to genetic mutations running in families and thus are referred to as familial.[14] To make matters more complex, mutations in a multitude of genes have been linked to familial ALS, most notably SOD1[15], FUS [16], and TDP-43[17]. Interestingly, TDP-43 inclusions are present in the cytoplasm of degenerating neurons in 97%of all ALS cases, including both familial and sporadic forms of the disease.[18] The discovery of the chromosome 9 open reading frame 72 (c9orf72) gene and its association to both familial and sporadic ALS has deepened our understanding of the processes triggering disease occurrence and progression.[19], [20] However, despite these advances, no definitive cause for ALS has been established.[5]
Frontotemporal dementia (FTD) is another neurological disorder characterized by personality and behavioral changes.[21] Typically, the disease manifests itself through the loss of spindle neurons in both the frontal and temporal lobes of the brain.[22] Because of this, many patients are often misdiagnosed with other illnesses such as depression or even Alzheimer’s disease.[23] While individual symptoms of FTD can be pharmacologically treated, there are no medications to cure or treat it. However, a number of drug candidates are being considered.[24] Recently, FTD and ALS have become characterized as a series of disorders that affect the size of the brain, particularly the frontal and anterior temporal lobes,[2] but the specific diagnosis varies depending on the area of the brain affected. Both FTD and ALS patients have been found to display a hexanucleotide repeat ‘GGGGCC’ expansion in the c9orf72 gene, pointing to a major role for this gene in ALS/FTD pathology.[25] This expansion is the most common genetic cause of both ALS and FTD.[26] Aside from C9orf72, other proteins are linked to FTD including FUS, Tau, and progranulin.[27]
1.2. Parkinson’s Disease
Parkinson’s Disease (PD) is a devastating neurodegenerative disease characterized by the loss of dopaminergic neurons of the substantia nigra pars compacta (SNPC).[4] The loss of SNPC neurons ultimately leads to a severe decrease in the amount of dopamine in the brain causing the patient to develop symptoms such as tremors, rigidity and overall poor motor coordination.[28] Currently, it is the second most common neurodegenerative disease found in developed nations, right behind Alzheimer’s disease.[29] Rather than treating the disease itself, PD treatments typically only ameliorate symptoms of the disease.[30], [31] These include antidepressants, anti-tremor, and dopamine promoting medications.[32] Sinemet (levodopa) is a dopamine promoter, and it is one of the more widely used treatments for PD. Sinemet results in many side effects including dizziness, nausea, heartburn, and even muscle pain, especially after prolonged use.[7] One particularly extreme side effect is dopamine dysregulation syndrome, where dopamine levels become so unstable that the patient ends up becoming addicted to dopamine replacement therapy use.[33] Hence, novel treatments are sorely needed.
Like ALS, most PD cases are sporadic. Only 10% of all PD cases are familial.[34] Several genes have been found to be associated with the onset of both familial and sporadic PD. Some of these include PARK8[35], LRRK2[36], and PINK1[35]. Prominently, the SNCA gene is associated with PD.[38], [39] This gene encodes for α-synuclein, the most notable component of Lewy bodies in degenerating dopaminergic neurons.[40], [41] Lewy bodies are proteinacious deposits which are a hallmark of PD, even appearing before PD symptoms begin to show.[42],[43]
1.3. Spinocerebellar and Friedrich’s Ataxias
Ataxias are usually defined as disorders where movement is hindered due to cerebral malfunctions.[44] Spinocerebellar ataxias (SCAs) are some of the most prevalent, accounting for nearly 36% of all ataxia cases in the United States.[45] The most commonly studied SCAs include SCA1, SCA3 (also known as Machado-Joseph disease), and SCA36.[46] Some symptoms include behavior or personality changes, muscle tremors, trouble walking, and slurred speech, reminiscent of those seen in ALS and FTD.[3] Proper diagnosis requires genetic testing; however, not all ataxias are able to be identified in this way.[47] There are at least 43 different types of ataxias, all with different symptoms, regions of localization, and identification markers; 11 of these are associated to repeat expansions in various genes.[48] For example, SCA1, SCA2, SCA3, SCA6, SCA7, and SCA17 all have CAG repeats in the ATXN1, ATXN2, ATXN3, CACNA1A, ATXN7, and TBP genes, respectively.[48] No reliable therapies have been developed for these diseases; however, trials using riluzole[49] and varenicline[50] have shown promising results.
Each SCA targets a different region of the brain, thus appearing with a different set of symptoms. SCA1 patients endure issues with movement and balance, such as numbness in their arms and legs that progressively increase over the years.[51] SCA3 has similar symptoms with the inclusion of involuntary eye movements in some patients, as well as sleeping problems.[52] SCA36 shares all of these symptoms; however, sleep deprivation is not as defined as an issue as it is in SCA3 patients. SCA36 has been linked to a GGCCTG hexanucleotide repeat expansion in the NOP56 gene.[53]
Another common ataxia is Friedreich’s ataxia (FRDA), an autosomal recessive disease with a typical onset before age 20, causing symptoms such as muscle weakness and deterioration, struggles with gait, and eventually death by cardiomyopathy.[54] Patients with the disease suffer from significant loss of both gray and white matter in the cerebellum and the dorsal medulla, leading to more severe symptoms and disease progression, depending on the amount lost.[55] The disease is linked to a triplet repeat expansion (GAA) in the FXN gene, leading to reduced expression of the frataxin protein in the mitochondria.[56] The frataxin protein is required for iron homeostasis inside cells of both eukaryotes and prokaryotes.[57] Iron-deficient anemia is a common symptom from FRDA that is caused by the depletion of frataxin, allowing for another route of possible treatment. Overall, FRDA can be managed through the use of medications targeting individual symptoms.[58]
1.4. Epigenetics and Histone Modifications
The term “epigenetic” refers to changes in gene activity occurring without direct changes in the DNA sequence.[59] These alterations are heritable, and many of them are reversible.[60] These edits occur at the molecular level and can be related to several factors such as environmental cues and stress.[61] While genetic factors greatly influence phenotype, epigenetic mechanisms add on a new layer of complexity to our understanding of biology.[62] For many disorders, epigenetics might hold the key to the development of disease; this is a particularly intriguing possibility for diseases with unknown etiology. Main epigenetic mechanisms include the methylation of DNA, microRNAs (miRNA) and the post-translational modification of histone proteins.[60]
In every nucleus, genomic DNA is packaged in a protein-DNA complex termed chromatin.[63] The basic unit of chromatin is called a nucleosome, consisting of a histone octamer comprising two copies of each of the core histone proteins (H2A, H2B, H3, and H4) around which DNA wraps.[64] Depending on how tightly associated the DNA is to the histone core, there is tightly packaged heterochromatin, or loosely packaged euchromatin.[65] Heterochromatin is transcriptionally silent, while euchromatin is actively transcribed.[66] The Ntermini of the core histones protrude out of the nucleosome, leaving them susceptible to a variety of post-translational modifications (PTMs) such as methylation, acetylation and phosphorylation.[67] Traditionally, it was thought that these modifications’ only role was to modulate the strength of the physical interactions between DNA and the histone octamer leading to alterations in gene transcription.[68] For instance, acetylation decreases the positive charge on histones and lessens the strength of the interaction with the negatively charged DNA backbone.[69] However, now we understand that these modifications also act as binding sites for other proteins. For example, H3K9me3 plays a strong role in silencing genes by serving as a binding platform for heterochromatin protein 1 (HP1).[70], [71] Furthermore, combinations of various histone modifications form a “code” that leads to different cellular outcomes.[72] For example, the monoubiquitination of H2BK123 leads to trimethylation of H3 on lysine 4 (H3K4me3) by activating the methylase complex COMPASS.[73] Histone PTMs play roles in nearly all biological processes including DNA repair,[74] gene transcription and translation[75] and even aging.[76]
Because of their impact on transcriptional activation and repression, one of the most studied histone modification is the methylation of lysine and arginine residues.[77] There are three levels of methylation, mono-, di-, and trimethylation, each responsible for a different effect on chromatin function, and controlled by distinct histone methyltransferases (HMTs) and demethylases.[78] The acetylation and deacetylation of histones can also result in changes in the structure of chromatin. Acetylation and deacetylation are controlled by acetyltransferases (HAT) and deacetylases (HDAC), respectively.[79] Acetylation usually occurs on lysine residues on the histone N-terminal tails.[80] The activation and repression of transcription through histone acetylation and deacetylation has led to the investigation of HDAC inhibitors as viable therapeutics. For instance, the use of sodium phenylbutyrate on mice with Huntington’s disease triggers neuroprotective effects.[81] Phosphorylation at serine, threonine and tyrosine residues on histone proteins has been associated to a wide range of cellular processes. For example, phosphorylation of Histone H2A(X) on serine 139 plays a significant role in the DNA damage response,[82] while H3S10ph is strongly associated to transcriptional activation.[83] Furthermore, H3S10ph has the ability to block methylation on H3K9 while increasing H4K16ac resulting in overall increased transcription.[66] H3S10ph is also correlated with increases in the levels of H3K14ac, highlighting crosstalk between these two marks.[83], [84]
Many histone PTMs have been correlated with various diseases. For instance, numerous cancer types, such as those from breast, bone, colon, and lung, display a loss of H4K16ac and H4K20me3 marks, as evidenced by mass spectrometry investigations.[85] Moreover, cells from patients with rheumatoid arthritis show an increase in H3K4me3 levels along with a reduction on the levels of H3K27me3, leading to believe that these marks play a role in the development of the disease.[86] Furthermore, certain genes believed to be involved in systemic lupus erythematosus display altered H3K4me3 levels.[87] The link between histone modifications and neurodegenerative disease is beginning to be discovered, with recent advances hinting at an important role for epigenetics in the etiology of ALS, FTD, PD, and ataxias.
2. HISTONE MODIFICATIONS IN NEURODEGENERATIVE DISEASE
2.1. Role of Histone PTMs in ALS
2.1.1. FUS/TLS
Fused in sarcoma (FUS; also known as TLS) is a protein found mostly in the nucleus, responsible for regulating transcription through alternative splicing, a process that occurs during gene expression that allows for a single gene to encode for several proteins.[88] FUS can also transport spliced mRNA out of the nucleus and into other organelles for further processing.[89] FUS is also thought to play a role in DNA repair, due to its ubiquitous presence at DNA repair sites.[90] The presence of the RGG-rich domain in FUS suggests it is able to aggregate and form droplets within the cell. RGG-rich domains are responsible for the aggregation of other proteins, such as LAF-1, and TDP-43.[91], [92] Indeed, FUS mislocalizes and aggregates in ALS, particularly in patients with FUS-associated familial ALS.[93] So far, more than 50 mutations in the FUS gene have been linked to ALS; many of these are concentrated in the region involved in DNA binding and mRNA processing.[94]
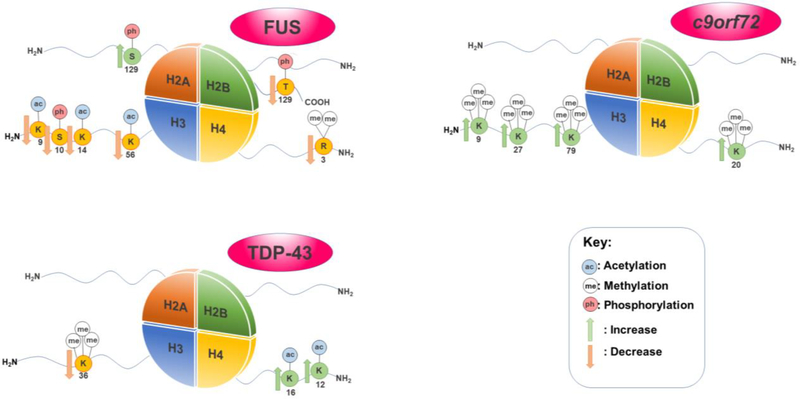
Several histone modifications have been found to be associated to the FUS protein in ALS (Figure 1).[95], [96] In a study of mutant or wild-type human FUS in motor neurons of murine spinal cord-dorsal root ganglion cultures, accumulation of FUS in the cytoplasm depleted the amount of PRMT1 available.[95] PRMT1 is an arginine methyltransferase responsible for mono- and asymmetric di-methylation of H4R3me2.[97] Sequestration of PRMT1 results in a decrease in H4R3me2, with accompanying decreases in H3K9ac, and H3K14ac resulting in loosened chromatin and increased access for the transcriptional machinery.[95] Furthermore, interaction between HDAC1 and FUS is needed for DNA repair.[98], [99] The presence of increased DNA damage is a hallmark of ALS and other neurodegenerative diseases.[100] Indeed, the lack of DNA damage response due to FUS aggregation is thought to cause neurodegeneration.[100] Exploiting a FUS overexpression yeast model, our own work revealed a severe decrease in the global levels of H3S10ph and H3K14ac.[96] In agreement with the results seen in the murine spinal cord-dorsal root ganglion cultures[91], H4R3me2 expression was also decreased in the yeast FUS model[96]. Furthermore, H3K56ac is also lowered in the yeast FUS proteinopathy model.[96] This modification is required to reinstate chromatin structure over repaired DNA and is critical for signaling completion of repair.[101]
Figure 1.
Notable histone modifications linked to FUS, TDP-43, and C9orf72 in ALS
2.1.2. TDP-43
TDP-43, or TAR DNA-binding domain protein, is another protein strongly linked to ALS. Interestingly, akin to FUS, TDP-43 is involved in certain aspects of RNA metabolism.[102] Residing mostly in the nucleus, TDP-43 contains a glycine-rich C-terminus, two RNA recognition motifs, and is capable of transporting proteins between the nucleus and the cytoplasm.[103], [104] TDP-43 is also associated with the formation of stress granules within cells, which are also thought to be linked to ALS.[105] Not surprisingly, mutations in TDP-43 have been extensively associated with neurodegeneration.[106]–[109] Misfolding of TDP-43 leads to a loss of protein function and a reduced ability to shuttle RNA. [110], [111] TDP-43 has been associated to a variety of histone modifications (Figure 1).[96] In a yeast TDP-43 overexpression model, our laboratory has found H3K36me3 to decrease. This same model also revealed an increase in H4K16ac and H4K12ac.[96] H4K16ac is a mark connected to DNA double strand break repair, a hallmark of ALS[112], [113], while H4K12ac, a mark associated with active promoters is a mark that has been associated to facilitating transcriptional elongation.[114], [115] H4K12ac has also been found to play a role in learning and memory[116], leading to believe that this mark plays a large role in the progression of memory problems in ALS. Interestingly, there are distinct changes in histone modification profiles for each proteinopathy model (FUS vs. TDP-43), providing evidence that histone PTM changes are not associated with general protein aggregation toxicity pathways, but specific proteinopathies.[96]
2.1.3. c9orf72
Discovered in 2011, the c9orf72 (chromosome 9 open reading frame 72) gene contains a hexanucleotide repeat expansion (HRE) ‘GGGGCC’. This HRE is the most common genetic cause of both ALS and FTD. [26] In general, the c9orf72 gene in healthy individuals contains up to 30 HRE; ALS and FTD patients have been found to carry over 500 repeats.[117] The number of HREs correlates to the severity of the symptoms, with a larger number of repeats decreasing the chances of survival for the patient.[118] This expansion is linked to ALS and FTD in multiple family pedigrees, being prominent in a number of familial ALS cases.[19], [119] The HRE are thought to lead to toxicity in a variety of ways including protein loss-of-function and gain-of-function toxicity through repeat-associated non-ATG (RAN) translation.[120] RAN translation is thought to lead to toxicity through the creation of short RNA transcripts that are prone to aggregation and can interfere with RNA processes.[120] These GGGGCC expanded repeats have been linked to histone modifications (Figure 1).[121] In particular, increases in the levels of H3K9me3, H3K27me3, H3K79me3, and H4K20me3 are associated to the expanded HREs in mutant c9orf72 and transcriptional repression of the gene.[121] Interestingly, the rise in trimethylation is not observed in normal c9orf72, and suggests a mechanism for loss-of-function toxicity in the mutant gene, as the protein product of c9orf72 has been recently implicated as an important protein in vesicle transportation and lysosomal biogenesis in motor neurons.[122] Furthermore, the decrease in the levels of these marks could be identified in the blood of a large cohort of ALS patients, suggesting that these histone PTMs could be potentially used as a diagnostic disease marker.[121]
2.2. Histone Post-Translational Modifications in FTD: FUS and Tau
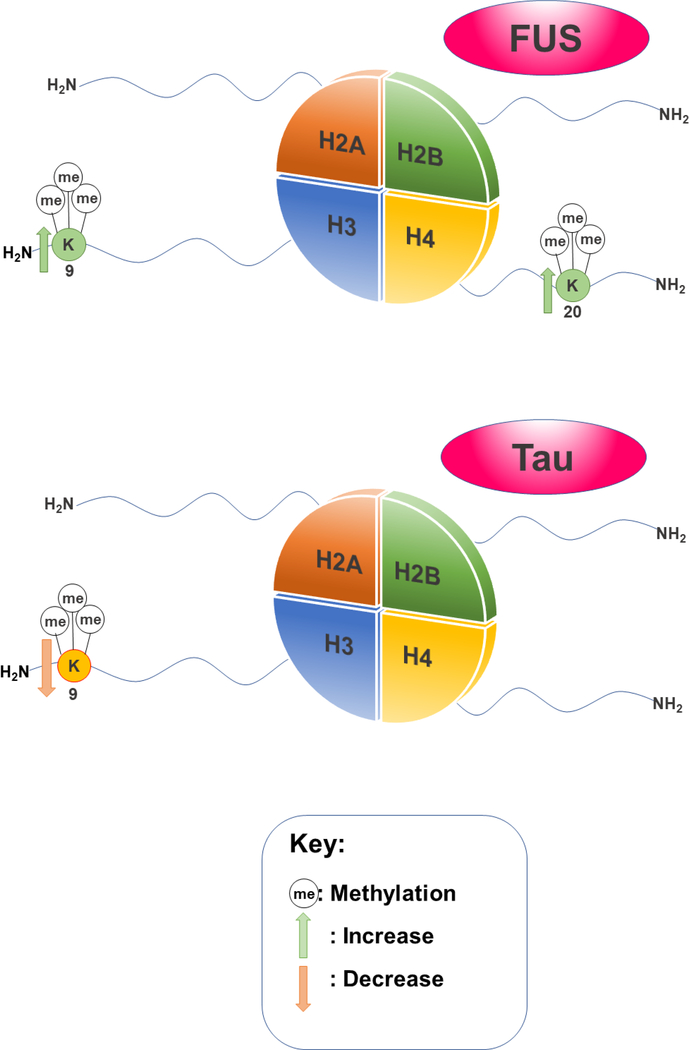
Many of the histone modification marks associated with FUS in ALS also seem to be found in FTD (Figure 2), regardless of the area of the brain that the disease may affect. In HeLa cells overexpressing FUS, the density of both trimethylation on H3K9 and trimethylation on H4K20 were progressively increased at telomeres.[123] The presence of H3K9me3 marks have been associated to binding heterochromatin protein 1 (HP1) to pericentric heterochromatin,[67] and therefore playing a role in transcriptional repression. Increased H3K9me3 marks have also been noted as relevant to DNA double strand break repair.[124] H4K20me3 is also responsible for transcriptional repression when present at promoters.[114] Transcriptional repression and DNA repair are mechanisms found in both ALS by FUS,[125] as well as in FTD by c9orf72.[121] As FUS, TDP-43 and C9orf72 are all involved in RNA processes and have been found to misfold in the context of disease, there is no doubt that a link between RNA disruption and proteostasis is associated to ALS and FTD.[126]
Figure 2.
Notable histone modifications linked to FUS and Tau in FTD
Tau is another protein that plays a role in the pathology of FTD. Tau is one of the first microtubule-associated proteins to be characterized.[127] Approximately 34 mutations in the Tau gene, MAPT, have been associated with FTD, alluding to a role in the onset of FTD.[128] Tau-deficient mice models display disruptions in the distribution and levels of H3K9me3 and HP1α, revealing lowered levels of H3K9 trimethylation (Figure 2).[129] In this context, histone modifications might also have a role outside of the nucleus. For instance, in the same mice models, H3K9me3 could not be incorporated into pericentromeric heterochromatin. Hence, H3K9me3 was only present in the cytoplasm of hippocampal neurons.[129] Cytoplasmic histones have been shown to impact cellular signaling and innate immunity.[130] This invokes the tantalizing possibility that histone PTMs occurring outside of the nucleus may be associated with disease onset related to Tau or other proteinopathies.
2.3. Histone Modifications in Parkinson’s Disease
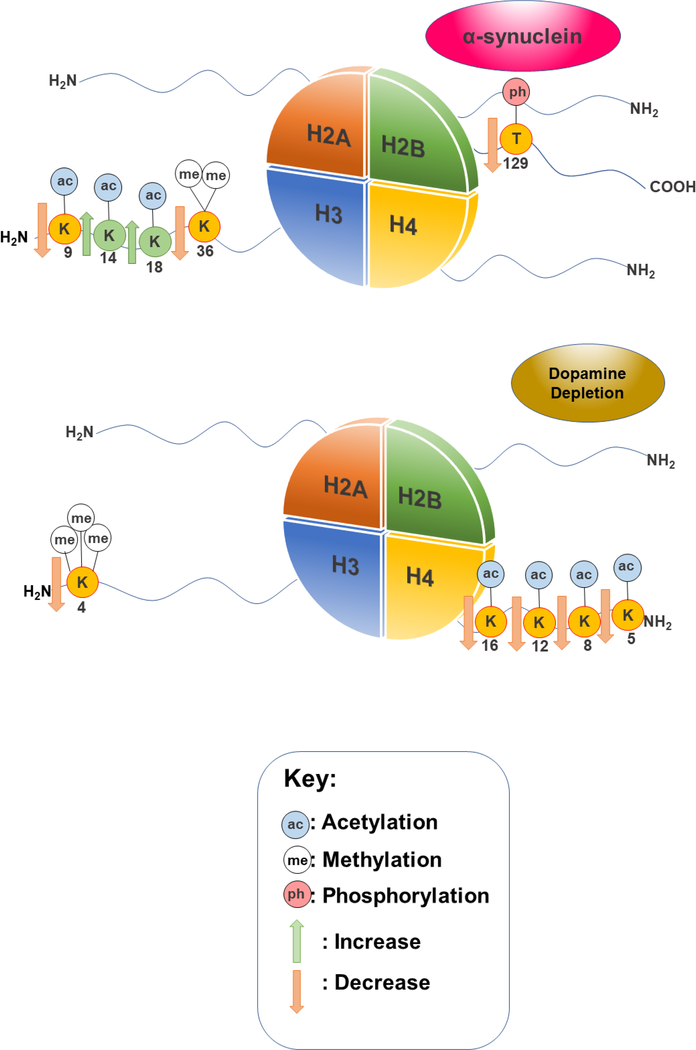
A variety of different epigenetic abnormalities have been associated to the pathology of PD.[131] Many studies have focused on the role of DNA methylation and microRNAs play in the development of PD; however histone modifications are beginning to gain traction as an attractive target of study. α-synuclein had been found to associate with histones through the inhibition of overall histone acetylation.[132] Furthermore, overexpression of α-synuclein is associated with lowered H3K9ac and increases in H3K14ac and H3K18ac marks in primary motor cortex cells.[133] H3K9ac is thought to have a role in active transcription, working in tandem with H3K14ac.[111], [112] α-synuclein overexpression was also shown to cause hypoacetylation of H3 in SH-SY5Y cells.[132] In a yeast model of α-synuclein proteinopathy, we find a decrease in the level of H2BT129ph, a mark linked to DNA damage.[96], [136] The same model revealed a decrease in the levels of H3K36me2.[96] H3K36me2 is a fairly well characterized histone mark that is known to play a role in DNA double-strand break repair.[137] Interestingly, dopamine depletion in PD has also been associated to histone PTMs. When dopamine levels decrease, a decrease in H3K4me3 is also observed in a mouse model. Moreover, histone deacetylation was observed at H4K5, H4K8, H4K12, and H4K16 in striatal cells from both mice and primate models treated with levodopa.[138] A summary of all these findings is presented in Figure 3.
Figure 3.
Notable histone modifications linked to α-synuclein and dopamine depletion in PD
2.4. Histone modifications in Spinocebrellar ataxia and Friedreich’s ataxia
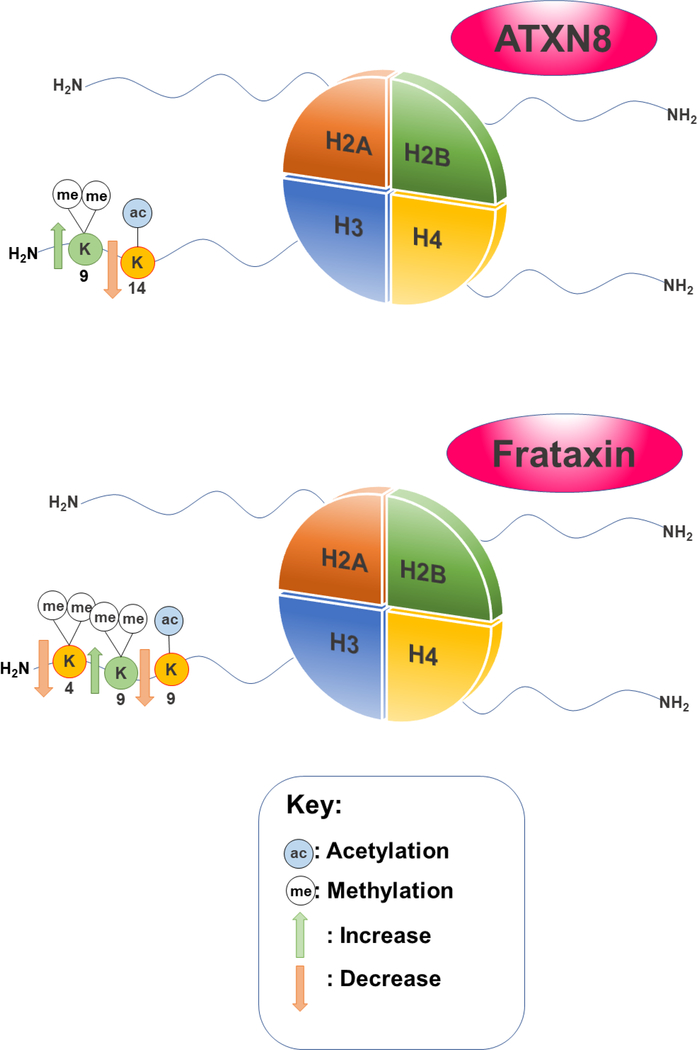
The variation in SCA types and their subsequent pathologies give rise to a vast landscape of investigation. As of yet, very few connections have been made between histone modifications and SCA, regardless of type. For instance, SCA8 is caused by both CTG/CAG combined repeats in the ATXN8 gene.[139] After studying a library of human kidney cell line variants with differing numbers of ATXN8 combined repeats, increased H3K9me2 expression was found in cells with 157 combined repeats, accompanied by a decrease in H3K14ac.[140] (Figure 4)
Figure 4.
Notable histone modifications linked to ATXN8 in SCA8 and Frataxin in FRDA
Several histone PTMs have been associated with FRDA (Figure 4). As previously mentioned, one of the main causes of FRDA is the triplet repeat expansion GAA responsible for the transcriptional silencing of the FXN gene.[141] Lymphoid cell lines from FRDA patients revealed that H3K4me2 and H3K4me marks decreased in the area surrounding the triplet repeat expansion when compared to unaffected cells.[142] Moreover, both human tissues and a transgenic mice model showed an overall decrease in H3K9ac levels accompanied by increases in H3K9me2 and H3K9me3 levels.[143] Collectively, these changes in histone marks would lead to lowered transcription, strongly suggesting that this is part of the mechanism that defines FRDA pathology.
3. HISTONE MODIFICATIONS IN NEURODEGENERATIVE DISEASE: NEW AVENUES FOR TREATMENT
HDAC inhibitors have been studied for potential use as therapies for ALS and FTD. [25] At present, butyrates are the most commonly studied class of HDAC inhibitors in humans, as they are able to easily penetrate the blood-brain barrier.[144] Sodium phenylbutyrate inhibits most HDACs, except for class III HDACs and class II HDAC6 and HDAC10.[145] In ALS mice models, treatment with sodium phenylbutyrate enhanced overall histone acetylation and improved survival rates.[144] Remarkably, in humans, sodium phenylbutyrate was not only safe and tolerable, but it was also able to increase histone acetylation in blood buffy-coat specimens while decreasing ALS symptoms.[146] Treatment of SOD1 G93A transgenic ALS mice with both riluzole and sodium phenylbutyrate increased H4 acetylation and survival by 21.5%, more than either riluzole or sodium phenylbutyrate on their own.[147] In the case of PD, administration of HDAC inhibitors in vivo or in vitro rescued α-synuclein- induced toxicity.[148] Despite their name, it is important to note that HDACs act upon several other non-histone targets.[149], [150] Therefore, there are other possible cellular mechanisms involving acetylation –independent of histones- by which HDAC inhibition can lead to reduction of toxicity. Nevertheless, combination of traditional treatments and treatments targeting epigenetic mechanisms might be in the not so distant future for ALS treatment.
In FRDA, treatment with nicotinamide (vitamin B3), an HDAC inhibitor, resulted in upregulation of the FXN gene by way of decreasing H3K9me3 and H3K27me3 at the FXN gene, and consequently increasing histone acetylation in both H3 and H4.[151] This revels a possible treatment for FRDA, especially when considering the widespread availability, tolerability and affordability of vitamin B3. In the case of SCA1 however, treatment with HDAC inhibitors may be inadequate. ATXN1 is the protein believed to be responsible for SCA1, and has been found to bind HDAC3 causing transcriptional repression in vitro[152], however depletion of HDAC3 does not change the SCA1 phenotype. Indeed, HDAC3 depletion did not decrease neurodegeneration in SCA1 transgenic mice, suggesting that HDAC inhibitors may not be a viable course of treatment.[153]
4. CONCLUDING REMARKS
In the past fifteen years, neurodegenerative diseases have been the subject of an explosion of research. Many advances have been made, but for patients, these diseases remain unstoppable and fatal, highlighting an immediate need for the development of more effective therapies. Histone post-translational modifications are highly druggable targets, and thus they represent an attractive target for pharmaceutical intervention. Aberrant histone modification profiles related to neurodegeneration are beginning to emerge and underscore the great need for the inclusion of the characterization of epigenetic mechanisms in neurodegenerative disease research. Increasing our understanding of the histone codes at play will allow us to envision viable approaches to restore proper chromatin dynamics and lead to new treatments for neurodegenerative diseases.
Highlights.
Cures for neurodegenerative disorders have yet to be discovered
Classical genetics fails to outline the pathology of neurodegenerative diseases
Epigenetic mechanisms such as the post-translational modification of histones may hold important clues to understanding disease progression.
Agents targeting epigenetic systems offer a promising approach for neurodegenerative disease treatment.
ACKNOWLEDGMENTS
Brooklyn College and an NIH NINDS Advanced Postdoctoral Career Transition Award (K22NS09131401) supported M.P.T. S.N.C. was supported by The Graduate Center of the City University of New York.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Gibson SB, Figueroa KP, Bromberg MB, Pulst S-M, and Cannon-Albright L, “Familial clustering of ALS in a population-based resource,” Neurology, vol. 82, no. 1, pp. 17–22, Jan. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ferrari R, Kapogiannis D, Huey ED, and Momeni P, “FTD and ALS: a tale of two diseases,” Curr. Alzheimer Res, vol. 8, no. 3, pp. 273–294, May 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Globas C et al. , “Early symptoms in spinocerebellar ataxia type 1, 2, 3, and 6,” Mov. Disord, vol. 23, no. 15, pp. 2232–2238. [DOI] [PubMed] [Google Scholar]
- [4].Poewe W et al. , “Parkinson disease,” Nat. Rev. Dis. Primer, vol. 3, p. 17013, Mar. 2017. [DOI] [PubMed] [Google Scholar]
- [5].Martin S, Al Khleifat A, and Al-Chalabi A, “What causes amyotrophic lateral sclerosis?,” F1000Research, vol. 6, Mar. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Yerbury JJ et al. , “Walking the tightrope: proteostasis and neurodegenerative disease,” J. Neurochem, vol. 137, no. 4, pp. 489–505. [DOI] [PubMed] [Google Scholar]
- [7].Hely MA, Morris JGL, Reid WGJ, and Trafficante R, “Sydney multicenter study of Parkinson’s disease: Non-L-dopa–responsive problems dominate at 15 years,” Mov. Disord, vol. 20, no. 2, pp. 190–199. [DOI] [PubMed] [Google Scholar]
- [8].Zarei S et al. , “A comprehensive review of amyotrophic lateral sclerosis,” Surg. Neurol. Int, vol. 6, Nov. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].“The Scottish Motor Neuron Disease Register: a prospective study of adult onset motor neuron disease in Scotland. Methodology, demography and clinical features of incident cases in 1989.,” J. Neurol. Neurosurg. Psychiatry, vol. 55, no. 7, pp. 536–541, Jul. 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Debove C, Zeisser P, Salzman PM, Powe LK, and Truffinet P, “The Rilutek (riluzole) Global Early Access Programme: an open-label safety evaluation in the treatment of amyotrophic lateral sclerosis,” Amyotroph. Lateral Scler. Mot. Neuron Disord. Off. Publ. World Fed. Neurol. Res. Group Mot. Neuron Dis, vol. 2, no. 3, pp. 153–158, Sep. 2001. [DOI] [PubMed] [Google Scholar]
- [11].“Edaravone in the Treatment of Amyotrophic Lateral Sclerosis: Efficacy and Access to Therapy - A Roundtable Discussion,” AJMC. [Online]. Available: http://www.ajmc.com/journals/supplement/2018/edaravone-treatment-als-access-totherapy/edaravone-treatment-of-als-efficacy-and-access-to-therapy-roundtable-discussion. [Accessed: 07-Jun-2018]. [PubMed]
- [12].Stevenson A et al. , “Riluzole protects against glutamate-induced slowing of neurofilament axonal transport,” Neurosci. Lett, vol. 454, no. 2, pp. 161–164, Apr. 2009. [DOI] [PubMed] [Google Scholar]
- [13].Cruz MP, “Edaravone (Radicava),” Pharm. Ther, vol. 43, no. 1, pp. 25–28, Jan. 2018. [PMC free article] [PubMed] [Google Scholar]
- [14].Al-Chalabi A, Jones A, Troakes C, King A, Al-Sarraj S, and van den Berg LH, “The genetics and neuropathology of amyotrophic lateral sclerosis,” Acta Neuropathol. (Berl.), vol. 124, no. 3, pp. 339–352, Sep. 2012. [DOI] [PubMed] [Google Scholar]
- [15].Gros-Louis F, Gaspar C, and Rouleau GA, “Genetics of familial and sporadic amyotrophic lateral sclerosis,” Biochim. Biophys. Acta, vol. 1762, no. 11–12, pp. 956–972, Dec. 2006. [DOI] [PubMed] [Google Scholar]
- [16].“ALS-associated mutant FUS induces selective motor neuron degeneration through toxic gain of function | Nature Communications.” [Online]. Available: https://www.nature.com/articles/ncomms10465. [Accessed: 27-May-2018]. [DOI] [PMC free article] [PubMed]
- [17].Scotter EL, Chen H-J, and Shaw CE, “TDP-43 Proteinopathy and ALS: Insights into Disease Mechanisms and Therapeutic Targets,” Neurotherapeutics, vol. 12, no. 2, pp. 352–363, Apr. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Arai T et al. , “TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis,” Biochem. Biophys. Res. Commun, vol. 351, no. 3, pp. 602–611, Dec. 2006. [DOI] [PubMed] [Google Scholar]
- [19].DeJesus-Hernandez M et al. , “Expanded GGGGCC Hexanucleotide Repeat in Noncoding Region of C9ORF72 Causes Chromosome 9p-Linked FTD and ALS,” Neuron, vol. 72, no. 2, pp. 245–256, Oct. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Renton AE et al. , “A Hexanucleotide Repeat Expansion in C9ORF72 Is the Cause of Chromosome 9p21-Linked ALS-FTD,” Neuron, vol. 72, no. 2, pp. 257–268, Oct. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Le Ber I et al. , “Demographic, neurological and behavioural characteristics and brain perfusion SPECT in frontal variant of frontotemporal dementia,” Brain J. Neurol, vol. 129, no. Pt 11, pp. 3051–3065, Nov. 2006. [DOI] [PubMed] [Google Scholar]
- [22].Santillo AF, Nilsson C, and Englund E, “von Economo neurones are selectively targeted in frontotemporal dementia,” Neuropathol. Appl. Neurobiol, vol. 39, no. 5, pp. 572–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Bott NT, Radke A, Stephens ML, and Kramer JH, “Frontotemporal dementia: diagnosis, deficits and management,” Neurodegener. Dis. Manag, vol. 4, no. 6, pp. 439–454, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Boxer AL et al. , “Frontotemporal Degeneration, the Next Therapeutic Frontier: Molecules and Animal Models for FTD drug development (Part 1 of 2 articles),” Alzheimers Dement. J. Alzheimers Assoc, vol. 9, no. 2, pp. 176–188, Mar. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Belzil VV, Katzman RB, and Petrucelli L, “ALS and FTD: an epigenetic perspective,” Acta Neuropathol. (Berl.), vol. 132, no. 4, pp. 487–502, Oct. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Fratta P et al. , “Screening a UK amyotrophic lateral sclerosis cohort provides evidence of multiple origins of the C9orf72 expansion,” Neurobiol. Aging, vol. 36, no. 1, pp. 546.e1546.e7, Jan. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Bennion Callister J and Pickering-Brown SM, “Pathogenesis/genetics of frontotemporal dementia and how it relates to ALS,” Exp. Neurol, vol. 262, pp. 84–90, Dec. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Triarhou LC, Dopamine and Parkinson’s Disease. Landes Bioscience, 2013. [Google Scholar]
- [29].Dexter DT and Jenner P, “Parkinson disease: from pathology to molecular disease mechanisms,” Free Radic. Biol. Med, vol. 62, pp. 132–144, Sep. 2013. [DOI] [PubMed] [Google Scholar]
- [30].Tabakman R, Lecht S, and Lazarovici P, “Neuroprotection by monoamine oxidase B inhibitors: a therapeutic strategy for Parkinson’s disease?,” BioEssays, vol. 26, no. 1, pp. 80–90. [DOI] [PubMed] [Google Scholar]
- [31].Jenner P and Langston JW, “Explaining ADAGIO: A critical review of the biological basis for the clinical effects of rasagiline,” Mov. Disord, vol. 26, no. 13, pp. 2316–2323. [DOI] [PubMed] [Google Scholar]
- [32].Fox SH, “Non-dopaminergic Treatments for Motor Control in Parkinson’s Disease,” Drugs, vol. 73, no. 13, pp. 1405–1415, Sep. 2013. [DOI] [PubMed] [Google Scholar]
- [33].O’Sullivan SS, Evans AH, and Lees AJ, “Dopamine Dysregulation Syndrome,” CNS Drugs, vol. 23, no. 2, pp. 157–170, Feb. 2009. [DOI] [PubMed] [Google Scholar]
- [34].Thomas B and Beal MF, “Parkinson’s disease,” Hum. Mol. Genet, vol. 16, no. R2, pp. R183–R194, Oct. 2007. [DOI] [PubMed] [Google Scholar]
- [35].Funayama M, Hasegawa K, Kowa H, Saito M, Tsuji S, and Obata F, “A new locus for Parkinson’s disease (PARK8) maps to chromosome 12p11.2-q13.1,” Ann. Neurol, vol. 51, no. 3, pp. 296–301, Mar. 2002. [DOI] [PubMed] [Google Scholar]
- [36].Gilks WP et al. , “A common LRRK2 mutation in idiopathic Parkinson’s disease,” The Lancet, vol. 365, no. 9457, pp. 415–416, Jan. 2005. [DOI] [PubMed] [Google Scholar]
- [37].Valente EM et al. , “Hereditary Early-Onset Parkinson’s Disease Caused by Mutations in PINK1,” Science, vol. 304, no. 5674, pp. 1158–1160, May 2004. [DOI] [PubMed] [Google Scholar]
- [38].Simón-Sánchez J et al. , “Genome-wide association study reveals genetic risk underlying Parkinson’s disease,” Nat. Genet, vol. 41, no. 12, pp. 1308–1312, Dec. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Chartier-Harlin M-C et al. , “α-synuclein locus duplication as a cause of familial Parkinson’s disease,” The Lancet, vol. 364, no. 9440, pp. 1167–1169, Sep. 2004. [DOI] [PubMed] [Google Scholar]
- [40].Polymeropoulos MH et al. , “Mutation in the α-Synuclein Gene Identified in Families with Parkinson’s Disease,” Science, vol. 276, no. 5321, pp. 2045–2047, Jun. 1997. [DOI] [PubMed] [Google Scholar]
- [41].Baba M et al. , “Aggregation of alpha-synuclein in Lewy bodies of sporadic Parkinson’s disease and dementia with Lewy bodies.,” Am. J. Pathol, vol. 152, no. 4, pp. 879–884, Apr. 1998. [PMC free article] [PubMed] [Google Scholar]
- [42].Gibb WR and Lees AJ, “The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease.,” J. Neurol. Neurosurg. Psychiatry, vol. 51, no. 6, pp. 745–752, Jun. 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Okazaki H, Lipkin LE, and Aronson SM, “Diffuse intracytoplasmic ganglionic inclusions (Lewy type) associated with progressive dementia and quadriparesis in flexion,” J. Neuropathol. Exp. Neurol, vol. 20, pp. 237–244, Apr. 1961. [DOI] [PubMed] [Google Scholar]
- [44].Akbar U and Ashizawa T, “Ataxia,” Neurol. Clin, vol. 33, no. 1, pp. 225–248, Feb. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Valera JM et al. , “Prevalence of spinocerebellar ataxia 36 in a US population,” Neurol. Genet, vol. 3, no. 4, Jul. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Paulson HL, “The Spinocerebellar Ataxias,” J. Neuro-Ophthalmol. Off. J. North Am. Neuro-Ophthalmol. Soc, vol. 29, no. 3, pp. 227–237, Sep. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Shakkottai VG and Fogel BL, “Autosomal Dominant Spinocerebellar Ataxia,” Neurol. Clin, vol. 31, no. 4, Nov. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Durr A, “Autosomal dominant cerebellar ataxias: polyglutamine expansions and beyond,” Lancet Neurol, vol. 9, no. 9, pp. 885–894, Sep. 2010. [DOI] [PubMed] [Google Scholar]
- [49].Ristori G et al. , “Riluzole in cerebellar ataxia: a randomized, double-blind, placebocontrolled pilot trial,” Neurology, vol. 74, no. 10, pp. 839–845, Mar. 2010. [DOI] [PubMed] [Google Scholar]
- [50].Zesiewicz TA et al. , “A randomized trial of varenicline (Chantix) for the treatment of spinocerebellar ataxia type 3,” Neurology, vol. 78, no. 8, pp. 545–550, Feb. 2012. [DOI] [PubMed] [Google Scholar]
- [51].Matilla-Dueñas A, Goold R, and Giunti P, “Clinical, genetic, molecular, and pathophysiological insights into spinocerebellar ataxia type 1,” Cerebellum Lond. Engl, vol. 7, no. 2, pp. 106–114, 2008. [DOI] [PubMed] [Google Scholar]
- [52].PAULSON H, “Machado-Joseph Disease/Spinocerebellar Ataxia Type 3,” Handb. Clin. Neurol. Ed. PJ Vinken GW Bruyn, vol. 103, pp. 437–449, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Ikeda Y et al. , “Clinical features of SCA36: a novel spinocerebellar ataxia with motor neuron involvement (Asidan),” Neurology, vol. 79, no. 4, pp. 333–341, Jul. 2012. [DOI] [PubMed] [Google Scholar]
- [54].Harding AE, “Friedreich’s ataxia: a clinical and genetic study of 90 families with an analysis of early diagnostic criteria and intrafamilial clustering of clinical features,” Brain J. Neurol, vol. 104, no. 3, pp. 589–620, Sep. 1981. [DOI] [PubMed] [Google Scholar]
- [55].Nave RD et al. , “Brain structural damage in Friedreich’s ataxia,” J. Neurol. Neurosurg. Psychiatry, vol. 79, no. 1, pp. 82–85, Jan. 2008. [DOI] [PubMed] [Google Scholar]
- [56].Campuzano V et al. , “Friedreich’s ataxia: autosomal recessive disease caused by an intronic GAA triplet repeat expansion,” Science, vol. 271, no. 5254, pp. 1423–1427, Mar. 1996. [DOI] [PubMed] [Google Scholar]
- [57].Bencze KZ et al. , “The Structure and Function of Frataxin,” Crit. Rev. Biochem. Mol. Biol, vol. 41, no. 5, pp. 269–291, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Mancuso M, Orsucci D, Choub A, and Siciliano G, “Current and emerging treatment options in the management of Friedreich ataxia,” Neuropsychiatr. Dis. Treat, vol. 6, pp. 491–499, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Deans C and Maggert KA, “What Do You Mean, ‘Epigenetic’?,” Genetics, vol. 199, no. 4, pp. 887–896, Apr. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Jablonka E and Lamb MJ, “Epigenetic Inheritance,” in International Encyclopedia of the Social & Behavioral Sciences, Smelser NJ and Baltes PB, Eds. Oxford: Pergamon, 2001, pp. 4706–4710. [Google Scholar]
- [61].Burggren W, “Epigenetic Inheritance and Its Role in Evolutionary Biology: Re-Evaluation and New Perspectives,” Biology, vol. 5, no. 2, May 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Peaston AE and Whitelaw E, “Epigenetics and phenotypic variation in mammals,” Mamm. Genome, vol. 17, no. 5, pp. 365–374, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Alberts B, Johnson A, Lewis J, Raff M, Roberts K, and Walter P, “Chromosomal DNA and Its Packaging in the Chromatin Fiber,” Mol. Biol. Cell 4th Ed., 2002. [Google Scholar]
- [64].Luger K and Richmond TJ, “The histone tails of the nucleosome,” Curr. Opin. Genet. Dev, vol. 8, no. 2, pp. 140–146, Apr. 1998. [DOI] [PubMed] [Google Scholar]
- [65].Elgin SC, “Heterochromatin and gene regulation in Drosophila,” Curr. Opin. Genet. Dev, vol. 6, no. 2, pp. 193–202, Apr. 1996. [DOI] [PubMed] [Google Scholar]
- [66].Grewal SIS and Moazed D, “Heterochromatin and Epigenetic Control of Gene Expression,” Science, vol. 301, no. 5634, pp. 798–802, Aug. 2003. [DOI] [PubMed] [Google Scholar]
- [67].Tessarz P and Kouzarides T, “Histone core modifications regulating nucleosome structure and dynamics,” Nat. Rev. Mol. Cell Biol, vol. 15, no. 11, pp. 703–708, Nov. 2014. [DOI] [PubMed] [Google Scholar]
- [68].Cedar H and Bergman Y, “Linking DNA methylation and histone modification: patterns and paradigms,” Nat. Rev. Genet, vol. 10, no. 5, pp. 295–304, May 2009. [DOI] [PubMed] [Google Scholar]
- [69].Bannister AJ and Kouzarides T, “Regulation of chromatin by histone modifications,” Cell Res, vol. 21, no. 3, pp. 381–395, Mar. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Barski A et al. , “High-resolution profiling of histone methylations in the human genome,” Cell, vol. 129, no. 4, pp. 823–837, May 2007. [DOI] [PubMed] [Google Scholar]
- [71].Lehnertz B et al. , “Suv39h-mediated histone H3 lysine 9 methylation directs DNA methylation to major satellite repeats at pericentric heterochromatin,” Curr. Biol. CB, vol. 13, no. 14, pp. 1192–1200, Jul. 2003. [DOI] [PubMed] [Google Scholar]
- [72].Jenuwein T, “Translating the Histone Code,” Science, vol. 293, no. 5532, pp. 1074–1080, Aug. 2001. [DOI] [PubMed] [Google Scholar]
- [73].Shilatifard A, “Chromatin Modifications by Methylation and Ubiquitination: Implications in the Regulation of Gene Expression,” Annu. Rev. Biochem, vol. 75, no. 1, pp. 243–269, 2006. [DOI] [PubMed] [Google Scholar]
- [74].Ünal E et al. , “DNA Damage Response Pathway Uses Histone Modification to Assemble a Double-Strand Break-Specific Cohesin Domain,” Mol. Cell, vol. 16, no. 6, pp. 991–1002, Dec. 2004. [DOI] [PubMed] [Google Scholar]
- [75].Karlić R, Chung H-R, Lasserre J, Vlahoviček K, and Vingron M, “Histone modification levels are predictive for gene expression,” Proc. Natl. Acad. Sci, vol. 107, no. 7, pp. 2926–2931, Feb. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Fraga MF and Esteller M, “Epigenetics and aging: the targets and the marks,” Trends Genet, vol. 23, no. 8, pp. 413–418, Aug. 2007. [DOI] [PubMed] [Google Scholar]
- [77].Greer EL and Shi Y, “Histone methylation: a dynamic mark in health, disease and inheritance,” Nat. Rev. Genet, vol. 13, no. 5, pp. 343–357, Apr. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Qureshi IA and Mehler MF, “Advances in Epigenetics and Epigenomics for Neurodegenerative Diseases,” Curr. Neurol. Neurosci. Rep, vol. 11, no. 5, pp. 464–473, Oct. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Peleg S, Feller C, Ladurner AG, and Imhof A, “The Metabolic Impact on Histone Acetylation and Transcription in Ageing,” Trends Biochem. Sci, vol. 41, no. 8, pp. 700–711, Aug. 2016. [DOI] [PubMed] [Google Scholar]
- [80].Kuo MH and Allis CD, “Roles of histone acetyltransferases and deacetylases in gene regulation,” BioEssays News Rev. Mol. Cell. Dev. Biol, vol. 20, no. 8, pp. 615–626, Aug. 1998. [DOI] [PubMed] [Google Scholar]
- [81].Gardian G et al. , “Neuroprotective effects of phenylbutyrate in the N171–82Q transgenic mouse model of Huntington’s disease,” J. Biol. Chem, vol. 280, no. 1, pp. 556–563, Jan. 2005. [DOI] [PubMed] [Google Scholar]
- [82].Rossetto D, Truman AW, Kron SJ, and Côté J, “Epigenetic modifications in doublestrand break DNA damage signaling and repair,” Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res, vol. 16, no. 18, pp. 4543–4552, Sep. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Lo WS et al. , “Phosphorylation of serine 10 in histone H3 is functionally linked in vitro and in vivo to Gcn5-mediated acetylation at lysine 14,” Mol. Cell, vol. 5, no. 6, pp. 917–926, Jun. 2000. [DOI] [PubMed] [Google Scholar]
- [84].Zippo A, Serafini R, Rocchigiani M, Pennacchini S, Krepelova A, and Oliviero S, “Histone Crosstalk between H3S10ph and H4K16ac Generates a Histone Code that Mediates Transcription Elongation,” Cell, vol. 138, no. 6, pp. 1122–1136, Sep. 2009. [DOI] [PubMed] [Google Scholar]
- [85].Fraga MF et al. , “Loss of acetylation at Lys16 and trimethylation at Lys20 of histone H4 is a common hallmark of human cancer,” Nat. Genet, vol. 37, no. 4, pp. 391–400, Apr. 2005. [DOI] [PubMed] [Google Scholar]
- [86].Araki Y et al. , “Histone Methylation and STAT-3 Differentially Regulate Interleukin-6– Induced Matrix Metalloproteinase Gene Activation in Rheumatoid Arthritis Synovial Fibroblasts,” Arthritis Rheumatol, vol. 68, no. 5, pp. 1111–1123. [DOI] [PubMed] [Google Scholar]
- [87].Dai Y, Zhang L, Hu C, and Zhang Y, “Genome-wide analysis of histone H3 lysine 4 trimethylation by ChIP-chip in peripheral blood mononuclear cells of systemic lupus erythematosus patients,” p. 11. [PubMed] [Google Scholar]
- [88].Ishigaki S et al. , “Position-dependent FUS-RNA interactions regulate alternative splicing events and transcriptions,” Sci. Rep, vol. 2, p. 529, Jul. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Zinszner H, Sok J, Immanuel D, Yin Y, and Ron D, “TLS (FUS) binds RNA in vivo and engages in nucleo-cytoplasmic shuttling,” J. Cell Sci, vol. 110 ( Pt 15), pp. 1741–1750, Aug. 1997. [DOI] [PubMed] [Google Scholar]
- [90].Sama RRK, Ward CL, and Bosco DA, “Functions of FUS/TLS From DNA Repair to Stress Response: Implications for ALS,” ASN NEURO, vol. 6, no. 4, Aug. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Elbaum-Garfinkle S et al. , “The disordered P granule protein LAF-1 drives phase separation into droplets with tunable viscosity and dynamics,” Proc. Natl. Acad. Sci. U. S. A, vol. 112, no. 23, pp. 7189–7194, Jun. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Dammer EB et al. , “Coaggregation of RNA-Binding Proteins in a Model of TDP-43 Proteinopathy with Selective RGG Motif Methylation and a Role for RRM1 Ubiquitination,” PLOS ONE, vol. 7, no. 6, p. e38658, Jun. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Mackenzie IRA et al. , “Pathological heterogeneity in amyotrophic lateral sclerosis with <Emphasis Type=“Italic”>FUS</Emphasis> mutations: two distinct patterns correlating with disease severity and mutation,” Acta Neuropathol. (Berl.), vol. 122, no. 1, pp. 87–98, Jul. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Deng H, Gao K, and Jankovic J, “The role of FUS gene variants in neurodegenerative diseases,” Nat. Rev. Neurol, vol. 10, no. 6, pp. 337–348, Jun. 2014. [DOI] [PubMed] [Google Scholar]
- [95].Tibshirani M et al. , “Cytoplasmic sequestration of FUS/TLS associated with ALS alters histone marks through loss of nuclear protein arginine methyltransferase 1,” Hum. Mol. Genet, vol. 24, no. 3, pp. 773–786, Feb. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Chen K et al. , “Neurodegenerative Disease Proteinopathies Are Connected to Distinct Histone Post-translational Modification Landscapes,” ACS Chem. Neurosci, vol. 9, no. 4, pp. 838–848, Apr. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Zhang X and Cheng X, “Structure of the Predominant Protein Arginine Methyltransferase PRMT1 and Analysis of Its Binding to Substrate Peptides,” Structure, vol. 11, no. 5, pp. 509–520, May 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Wang W-Y et al. , “Interaction of FUS and HDAC1 Regulates DNA Damage Response and Repair in Neurons,” Nat. Neurosci, vol. 16, no. 10, pp. 1383–1391, Oct. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Singh I et al. , “High mobility group protein-mediated transcription requires DNA damage marker γ-H2AX,” Cell Res, vol. 25, no. 7, pp. 837–850, Jul. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Naumann M et al. , “Impaired DNA damage response signaling by FUS-NLS mutations leads to neurodegeneration and FUS aggregate formation,” Nat. Commun, vol. 9, no. 1, p. 335, Jan. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Chen C-C et al. , “Acetylated Lysine 56 on Histone H3 Drives Chromatin Assembly after Repair and Signals for the Completion of Repair,” Cell, vol. 134, no. 2, pp. 231–243, Jul. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Buratti E and Baralle FE, “TDP-43: gumming up neurons through protein-protein and protein-RNA interactions,” Trends Biochem. Sci, vol. 37, no. 6, pp. 237–247, Jun. 2012. [DOI] [PubMed] [Google Scholar]
- [103].Buratti E, Brindisi A, Giombi M, Tisminetzky S, Ayala YM, and Baralle FE, “TDP-43 binds heterogeneous nuclear ribonucleoprotein A/B through its C-terminal tail: an important region for the inhibition of cystic fibrosis transmembrane conductance regulator exon 9 splicing,” J. Biol. Chem, vol. 280, no. 45, pp. 37572–37584, Nov. 2005. [DOI] [PubMed] [Google Scholar]
- [104].Winton MJ, Igaz LM, Wong MM, Kwong LK, Trojanowski JQ, and Lee VM-Y, “Disturbance of Nuclear and Cytoplasmic TAR DNA-binding Protein (TDP-43) Induces Disease-like Redistribution, Sequestration, and Aggregate Formation,” J. Biol. Chem, vol. 283, no. 19, pp. 13302–13309, May 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Aulas A and Vande Velde C, “Alterations in stress granule dynamics driven by TDP-43 and FUS: a link to pathological inclusions in ALS?,” Front. Cell. Neurosci, vol. 9, p. 423, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Lagier-Tourenne C, Polymenidou M, and Cleveland DW, “TDP-43 and FUS/TLS: emerging roles in RNA processing and neurodegeneration,” Hum. Mol. Genet, vol. 19, no. R1, pp. R46–R64, Apr. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Kabashi E et al. , “TARDBP mutations in individuals with sporadic and familial amyotrophic lateral sclerosis,” Nat. Genet, vol. 40, no. 5, pp. 572–574, May 2008. [DOI] [PubMed] [Google Scholar]
- [108].Van Deerlin VM et al. , “TARDBP mutations in amyotrophic lateral sclerosis with TDP-43 neuropathology: a genetic and histopathological analysis,” Lancet Neurol, vol. 7, no. 5, pp. 409–416, May 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Gitcho MA et al. , “TDP-43 A315T Mutation in Familial Motor Neuron Disease,” Ann. Neurol, vol. 63, no. 4, pp. 535–538, Apr. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Alami NH et al. , “Axonal transport of TDP-43 mRNA granules in neurons is impaired by ALS-causing mutations,” Neuron, vol. 81, no. 3, pp. 536–543, Feb. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Liu-Yesucevitz L et al. , “ALS-Linked Mutations Enlarge TDP-43-Enriched Neuronal RNA Granules in the Dendritic Arbor,” J. Neurosci, vol. 34, no. 12, pp. 4167–4174, Mar. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Li L and Wang Y, “Cross-talk between the H3K36me3 and H4K16ac histone epigenetic marks in DNA double-strand break repair,” J. Biol. Chem, vol. 292, no. 28, pp. 11951–11959, 14 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Coppedè F, “An Overview of DNA Repair in Amyotrophic Lateral Sclerosis,” Sci. World J, vol. 11, pp. 1679–1691, Oct. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Wang Z et al. , “Combinatorial patterns of histone acetylations and methylations in the human genome,” Nat. Genet, vol. 40, no. 7, pp. 897–903, Jul. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Cho H et al. , “A Human RNA Polymerase II Complex Containing Factors That Modify Chromatin Structure,” Mol. Cell. Biol, vol. 18, no. 9, pp. 5355–5363, Sep. 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Peleg S et al. , “Altered Histone Acetylation Is Associated with Age-Dependent Memory Impairment in Mice,” Science, vol. 328, no. 5979, pp. 753–756, May 2010. [DOI] [PubMed] [Google Scholar]
- [117].Beck J et al. , “Large C9orf72 Hexanucleotide Repeat Expansions Are Seen in Multiple Neurodegenerative Syndromes and Are More Frequent Than Expected in the UK Population,” Am. J. Hum. Genet, vol. 92, no. 3, pp. 345–353, Mar. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].van Blitterswijk M et al. , “Association between repeat sizes and clinical and pathological characteristics in carriers of C9ORF72 repeat expansions (Xpansize-72): a cross-sectional cohort study,” Lancet Neurol., vol. 12, no. 10, pp. 978–988, Oct. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].van Blitterswijk M, DeJesus-Hernandez M, and Rademakers R, “How do C9ORF72 repeat expansions cause ALS and FTD: can we learn from other non-coding repeat expansion disorders?,” Curr. Opin. Neurol, vol. 25, no. 6, pp. 689–700, Dec. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Paez-Colasante X, Figueroa-Romero C, Sakowski SA, Goutman SA, and Feldman EL, “Amyotrophic lateral sclerosis: mechanisms and therapeutics in the epigenomic era,” Nat. Rev. Neurol, vol. 11, no. 5, pp. 266–279, May 2015. [DOI] [PubMed] [Google Scholar]
- [121].Belzil VV et al. , “Reduced C9orf72 gene expression in c9FTD/ALS is caused by histone trimethylation, an epigenetic event detectable in blood,” Acta Neuropathol. (Berl.), vol. 126, no. 6, pp. 895–905, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Shi Y et al. , “Haploinsufficiency leads to neurodegeneration in C9ORF72 ALS/FTD human induced motor neurons,” Nat. Med, vol. 24, no. 3, pp. 313–325, Mar. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Takahama K et al. , “Regulation of Telomere Length by G-Quadruplex Telomere DNA- and TERRA-Binding Protein TLS/FUS,” Chem. Biol, vol. 20, no. 3, pp. 341–350, Mar. 2013. [DOI] [PubMed] [Google Scholar]
- [124].Ayrapetov MK, Gursoy-Yuzugullu O, Xu C, Xu Y, and Price BD, “DNA double-strand breaks promote methylation of histone H3 on lysine 9 and transient formation of repressive chromatin,” Proc. Natl. Acad. Sci. U. S. A, vol. 111, no. 25, pp. 9169–9174, Jun. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Walker C and El-Khamisy SF, “Perturbed autophagy and DNA repair converge to promote neurodegeneration in amyotrophic lateral sclerosis and dementia,” Brain, vol. 141, no. 5, pp. 1247–1262, May 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Ling S-C, Polymenidou M, and Cleveland DW, “Converging Mechanisms in ALS and FTD: Disrupted RNA and Protein Homeostasis,” Neuron, vol. 79, no. 3, pp. 416–438, Aug. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Drubin DG and Kirschner MW, “Tau protein function in living cells,” J. Cell Biol, vol. 103, no. 6 Pt 2, pp. 2739–2746, Dec. 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Rademakers R, Cruts M, and van Broeckhoven C, “The role of tau (MAPT) in frontotemporal dementia and related tauopathies,” Hum. Mutat, vol. 24, no. 4, pp. 277–295. [DOI] [PubMed] [Google Scholar]
- [129].Mansuroglu Z et al. , “Loss of Tau protein affects the structure, transcription and repair of neuronal pericentromeric heterochromatin,” Sci. Rep, vol. 6, Sep. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Parseghian MH and Luhrs KA, “Beyond the walls of the nucleus: the role of histones in cellular signaling and innate immunity,” Biochem. Cell Biol. Biochim. Biol. Cell, vol. 84, no. 4, pp. 589–604, Aug. 2006. [DOI] [PubMed] [Google Scholar]
- [131].Feng Y, Jankovic J, and Wu Y-C, “Epigenetic mechanisms in Parkinson’s disease,” J. Neurol. Sci, vol. 349, no. 1, pp. 3–9, Feb. 2015. [DOI] [PubMed] [Google Scholar]
- [132].Kontopoulos E, Parvin JD, and Feany MB, “α-synuclein acts in the nucleus to inhibit histone acetylation and promote neurotoxicity,” Hum. Mol. Genet, vol. 15, no. 20, pp. 3012–3023, Oct. 2006. [DOI] [PubMed] [Google Scholar]
- [133].Gebremedhin KG and Rademacher DJ, “Histone H3 acetylation in the postmortem Parkinson’s disease primary motor cortex,” Neurosci. Lett, vol. 627, pp. 121–125, Aug. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Karmodiya K, Krebs AR, Oulad-Abdelghani M, Kimura H, and Tora L, “H3K9 and H3K14 acetylation co-occur at many gene regulatory elements, while H3K14ac marks a subset of inactive inducible promoters in mouse embryonic stem cells,” BMC Genomics, vol. 13, p. 424, Aug. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Gates LA et al. , “Acetylation on histone H3 lysine 9 mediates a switch from transcription initiation to elongation,” J. Biol. Chem, vol. 292, no. 35, pp. 14456–14472, 01 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Kyriss MNM, Jin Y, Gallegos IJ, Sanford JA, and Wyrick JJ, “Novel functional residues in the core domain of histone H2B regulate yeast gene expression and silencing and affect the response to DNA damage,” Mol. Cell. Biol, vol. 30, no. 14, pp. 3503–3518, Jul. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Fnu S et al. , “Methylation of histone H3 lysine 36 enhances DNA repair by nonhomologous end-joining,” Proc. Natl. Acad. Sci, vol. 108, no. 2, pp. 540–545, Jan. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Nicholas AP et al. , “Striatal histone modifications in models of levodopa-induced dyskinesia,” J. Neurochem, vol. 106, no. 1, pp. 486–494. [DOI] [PubMed] [Google Scholar]
- [139].Koob MD et al. , “An untranslated CTG expansion causes a novel form of spinocerebellar ataxia (SCA8),” Nat. Genet, vol. 21, no. 4, pp. 379–384, Apr. 1999. [DOI] [PubMed] [Google Scholar]
- [140].Chen I-C et al. , “Spinocerebellar ataxia type 8 larger triplet expansion alters histone modification and induces RNA foci,” BMC Mol. Biol, vol. 10, p. 9, Feb. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Li Y et al. , “Expanded GAA repeats impede transcription elongation through the FXN gene and induce transcriptional silencing that is restricted to the FXN locus,” Hum. Mol. Genet, vol. 24, no. 24, pp. 6932–6943, Dec. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Kim E, Napierala M, and Dent SYR, “Hyperexpansion of GAA repeats affects post-initiation steps of FXN transcription in Friedreich’s ataxia,” Nucleic Acids Res, vol. 39, no. 19, pp. 8366–8377, Oct. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Al-Mahdawi S et al. , “The Friedreich ataxia GAA repeat expansion mutation induces comparable epigenetic changes in human and transgenic mouse brain and heart tissues,” Hum. Mol. Genet, vol. 17, no. 5, pp. 735–746, Mar. 2008. [DOI] [PubMed] [Google Scholar]
- [144].Ryu H et al. , “Sodium phenylbutyrate prolongs survival and regulates expression of antiapoptotic genes in transgenic amyotrophic lateral sclerosis mice,” J. Neurochem, vol. 93, no. 5, pp. 1087–1098. [DOI] [PubMed] [Google Scholar]
- [145].Davie JR, “Inhibition of histone deacetylase activity by butyrate,” J. Nutr, vol. 133, no. 7 Suppl, pp. 2485S–2493S, 2003. [DOI] [PubMed] [Google Scholar]
- [146].Cudkowicz ME et al. , “Phase 2 study of sodium phenylbutyrate in ALS,” Amyotroph. Lateral Scler. Off. Publ. World Fed. Neurol. Res. Group Mot. Neuron Dis, vol. 10, no. 2, pp. 99–106, Apr. 2009. [DOI] [PubMed] [Google Scholar]
- [147].Signore SJD et al. , “Combined riluzole and sodium phenylbutyrate therapy in transgenic amyotrophic lateral sclerosis mice,” Amyotroph. Lateral Scler, vol. 10, no. 2, pp. 85–94, Jan. 2009. [DOI] [PubMed] [Google Scholar]
- [148].Kontopoulos E, Parvin JD, and Feany MB, “Alpha-synuclein acts in the nucleus to inhibit histone acetylation and promote neurotoxicity,” Hum. Mol. Genet, vol. 15, no. 20, pp. 3012–3023, Oct. 2006. [DOI] [PubMed] [Google Scholar]
- [149].Luo J, Su F, Chen D, Shiloh A, and Gu W, “Deacetylation of p53 modulates its effect on cell growth and apoptosis,” Nature, vol. 408, no. 6810, pp. 377–381, Nov. 2000. [DOI] [PubMed] [Google Scholar]
- [150].Yang W-M, Yao Y-L, Sun J-M, Davie JR, and Seto E, “Isolation and Characterization of cDNAs Corresponding to an Additional Member of the Human Histone Deacetylase Gene Family,” J. Biol. Chem, vol. 272, no. 44, pp. 28001–28007, Oct. 1997. [DOI] [PubMed] [Google Scholar]
- [151].Chan PK et al. , “Heterochromatinization induced by GAA-repeat hyperexpansion in Friedreich’s ataxia can be reduced upon HDAC inhibition by vitamin B3,” Hum. Mol. Genet, vol. 22, no. 13, pp. 2662–2675, Jul. 2013. [DOI] [PubMed] [Google Scholar]
- [152].Tsai C-C et al. , “Ataxin 1, a SCA1 neurodegenerative disorder protein, is functionally linked to the silencing mediator of retinoid and thyroid hormone receptors,” Proc. Natl. Acad. Sci, vol. 101, no. 12, pp. 4047–4052, Mar. 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Venkatraman A et al. , “The histone deacetylase HDAC3 is essential for Purkinje cell function, potentially complicating the use of HDAC inhibitors in SCA1,” Hum. Mol. Genet, vol. 23, no. 14, pp. 3733–3745, Jul. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]