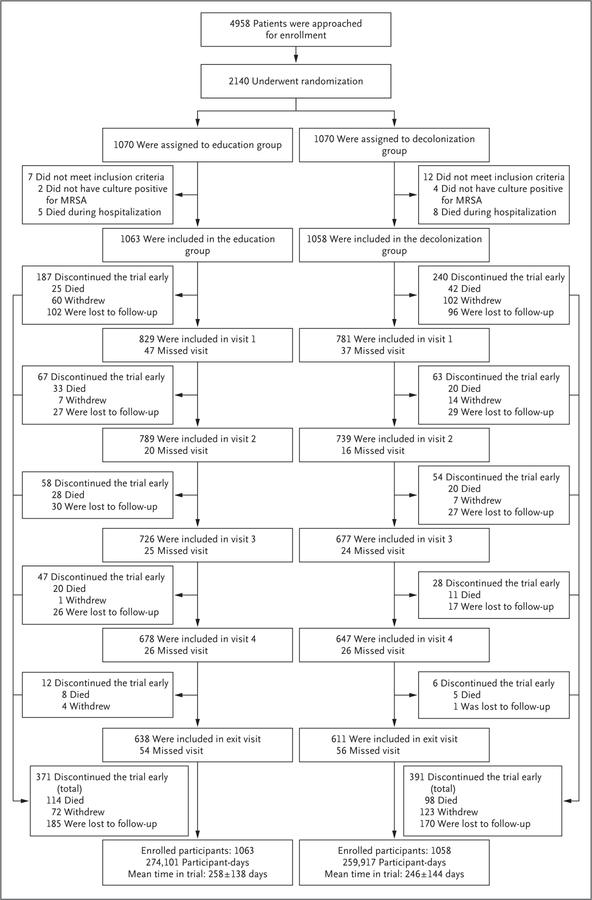
Figure 1. Randomization and Follow-up of the Participants.
This flow chart describes the recruitment and the four follow-up visits (at 1, 3, 6, and 9 months) for the 1-year period after hospital discharge. Recruitment occurred during hospitalization, and 19 participants were excluded from the postdischarge trial population because they did not meet inclusion criteria, leaving 2121 participants in the per-protocol population (1063 participants in the education group and 1058 in the decolonization group). Early exit from the trial was provided between each visit and included active withdrawal from the trial, loss to follow-up, and death. Active withdrawal represented situations in which participants indicated their desire to withdraw from the trial. Loss to follow-up was defined as the inability to contact the participant for 3 months, at which point the participant was removed from the trial at the time of last contact. Visits indicate both participants who successfully completed the visit and those who remained in the trial but missed that visit. The mean (±SD) time in the trial (in days) is shown for each group. All deaths were considered by the investigators to be unrelated to side effects from decolonization products. Summary boxes are provided at the bottom of the figure. MRSA denotes methicillin-resistant Staphylococcus aureus.