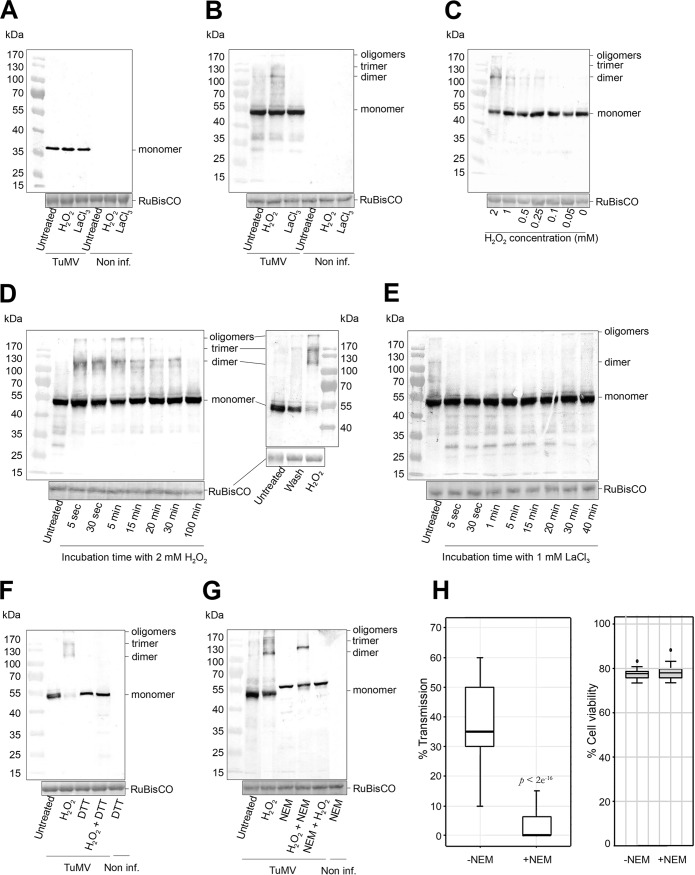
FIG 4.
Nonreducing SDS-PAGE/Western blotting of HC-Pro and CP from TuMV-infected turnip protoplasts. The samples were lysed in a buffer without reducing agents to conserve the disulfide bridges. H2O2 and LaCl3 treatments did not modify the migration profile of the capsid protein (CP) (A), whereas they induced (H2O2) or inhibited (LaCl3) formation of HC-Pro oligomers (B). The concentration range and the kinetics of H2O2 incubation show that HC-Pro oligomerization was induced by a minimum concentration of 0.25 mM (C) and that it was rapid and reversible, either by extended H2O2 treatment (left) or by washing protoplasts (right) (D). (E) Inhibition of HC-Pro oligomerization by LaCl3 was also rapid and reversible. HC-Pro oligomers were formed by intermolecular disulfide bridges, because incubation of protoplasts with DTT, either alone or after H2O2 treatment, abolished HC-Pro oligomers (F), and treatment with NEM before but not after previous incubation with H2O2 prevented their formation (G). (H, left) Transmission tests using NEM-treated protoplasts show a drastic diminution of TuMV transmission. Transmission tests were performed three times using 320 plants per condition and analyzed by generalized linear models as described in Fig. 1. (Right) Protoplast viability assays show that NEM treatment did not change cell viability under the conditions used. TuMV, samples of TuMV-infected protoplasts; Non inf., samples of noninfected protoplasts; LaCl3, treatment with 1 mM LaCl3 for 5 min; H2O2, treatment with 2 mM H2O2 for 5 min; wash, H2O2 was removed by centrifugation and resuspension of protoplasts in fresh medium; DTT, treatment with 5 mM DTT for 30 min; NEM, treatment with 3 mM NEM for 20 min. Equal loading of lanes is shown by Ponceau S red staining of the large RuBisCO subunit. A precolored ladder and the molecular masses in kilodaltons are indicated at one side of each blot. P value in panel H obtained by generalized linear models from three independent experiments.