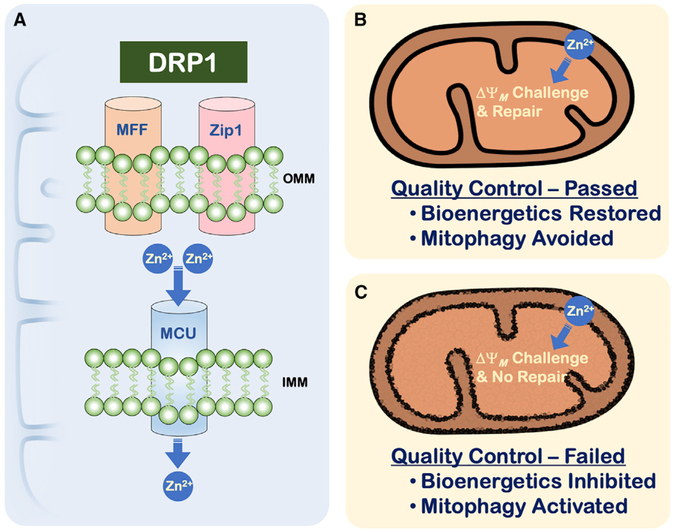
Figure 1. The DRP1-Zip1 Interaction Initiates a Mitochondrial Quality Control Surveillance Program.
(A) DRP1 re-localizes from the cytosol to the OMM by binding to a docking protein, MFF. Once at the OMM, DRP1 associates with Zip1, which promotes the entry of Zn2+ into the matrix via a MCU-dependent manner. The mechanism by which DRP1 activates Zip1 to stimulate MCU activity remains unclear. There are two proposed outcomes from this pathway (B and C).
(B) Upon Zn2+ entry into mitochondria, components of the electron transport chain are inhibited, resulting in decreased ΔΨM. Normal mitochondrial function (e.g., responsive changes to nuclear-mitochondrial signaling to re-establish ΔΨM) allows for the rapid recovery of ΔΨM and protection against mitophagy.
(C) Alternatively, Zn2+-induced inhibition of ΔΨM is not repaired, and the mitochondrion is destined for mitophagy. This mechanism may identify distinct organelles or regions of the mitochondrial network that fail to recover from bioenergetic stress due to aberrations in mitochondrial gene expression, coordinated nuclear-mitochondrial signaling, or compromised electron transport chain assembly.