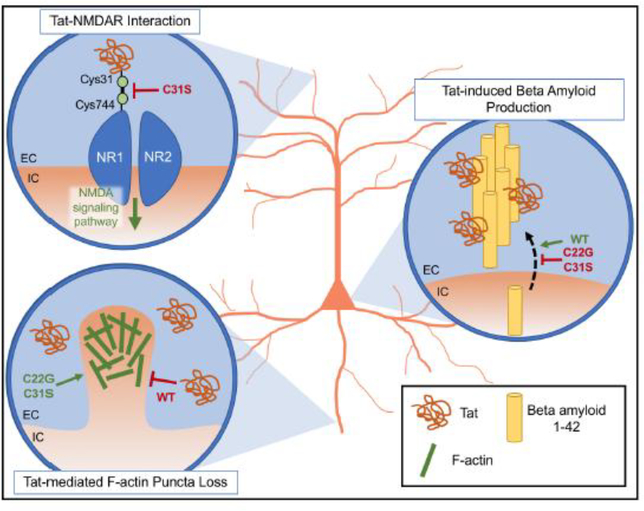
Fig. 4. Proposed model of the effect of cysteine-rich domain HIV-1 Tat variants on Tat-mediated neuronal neurotoxicity.
Overview of HIV-1 Tat variants that produce neurotoxic effects differing from wild-type (WT) Tat. (Top left) Interaction between Tat Cys31 and NMDAR NR1 Cys744. Excitotoxicity generated by the formation of a disulfide bond between WT Tat Cys31 and Cys744 of the NMDAR NR1 subunit is reduced with the replacement of Tat Cys31 with serine. This is presumably due to the inability of Tat C31S to form this disulfide bond. (Bottom left) Tat variants modulate F-actin puncta loss in neuronal dendrites. Although the mechanism of Tat-mediated synaptodendritic injury is not fully understood, treatment of primary hippocampal neurons with WT Tat results in decreased F-actin puncta, the presence of which normally indicates pre- and post-synaptic structural integrity. The Tat-mediated decrease in F-actin puncta is not seen upon treatment with C22G or C31S. (Right) Amyloid beta production is altered by exposure to Tat variants. Treatment of primary hippocampal neurons with WT Tat promoted beta amyloid 1–42 production and release into cell culture supernatant, where it may aggregate and form stiff fibrils in the presence of WT Tat. The introduction of the C22G or C31S variant restored beta amyloid 1–42 production to control levels. EC, extracellular; IC, intracellular.