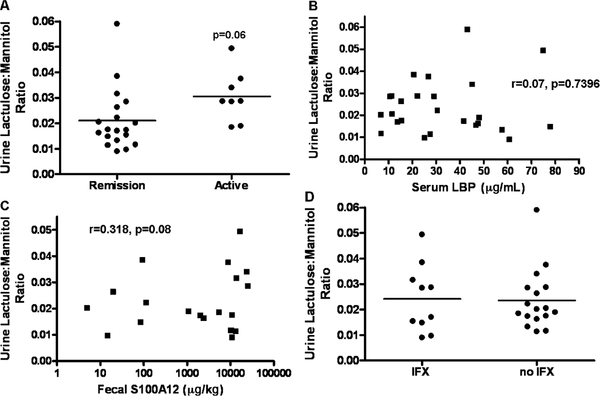
FIGURE 3.
Urine lactulose:mannitol ratio and clinical disease activity, systemic and mucosal inflammation, and infliximab exposure. Healthy controls (n = 15) and CD patients (n = 27) ingested a mixture of sucrose, lactulose, and mannitol, and urinary concentrations of lactulose and mannitol were determined by high-pressure liquid chromatography. A, CD patients were stratified by clinical disease activity. The relationship between (B) serum LBP and (C) fecal S100A12 and urine lactulose:mannitol ratio is shown. D, CD patients were stratified by infliximab exposure at the time of urine lactulose:mannitol measurement. Data are shown as the urinary lactulose:mannitol excretion ratio, with the means for each group as indicated in (A) and (D). CD = Crohn disease.