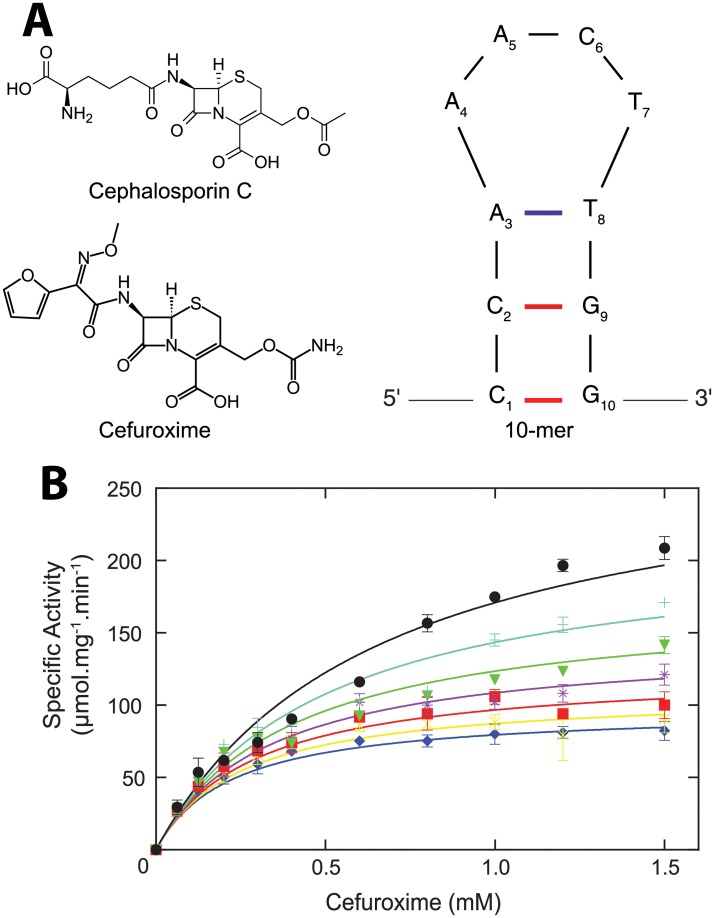
Fig 1. Inhibition of 5/B/6 MBL by the 10-mer aptamer during cefuroxime hydrolysis.
A) Chemical structures of the β-lactam antibiotics cephalosporin C and cefuroxime. Also shown is the M-fold predicted secondary structure of the 10-mer DNA aptamer. B) Plot of the specific activity versus cefuroxime concentration. Points and error bars are the average and standard deviation of at least three measurements. Solid lines represent the non-linear regression calculation (global correlation coefficient R2 = 0.964), which shows the uncompetitive inhibition pattern during cefuroxime hydrolysis. Black circle: 10-mer concentration [I] = 0 (local correlation coefficient R2 = 0.969); cyan: [I] = 20 nM (R2 = 0.957); green: [I] = 40 nM (R2 = 0.961); magenta: [I] = 60 nM (R2 = 0.965); red: [I] = 80 nM (R2 = 0.949); yellow: [I] = 100 nM (R2 = 0.913); and blue: [I] = 120 nM (R2 = 0.960).