Summary
Animal navigation relies on the available environmental cues and, where present, visual cues typically dominate. While much is known about vision-assisted navigation, knowledge of navigation in the dark is scarce. Here, we combine individual tracking, dynamic modular nest structures, and spatially resolved chemical profiling to study how Camponotus fellah ants navigate within the dark labyrinth of their nest. We find that, contrary to ant navigation above ground, underground navigation cannot rely on long-range information. This limitation emphasizes the ants' capabilities associated with other navigational strategies. Indeed, apart from gravity, underground navigation relies on self-referenced memories of multiple locations and on socially generated chemical cues placed at decision points away from the target. Moreover, the ants quickly readjust the weights attributed to these information sources in response to environmental changes. Generally, studying well-known behaviors in a variety of environmental contexts holds the potential of revealing new insights into animal cognition.
Subject Areas: Wildlife Behavior, Biological Sciences, Entomology
Graphical Abstract

Highlights
-
•
We combine multiple technologies to study how ants navigate within their dark nest
-
•
Ants substitute visual cues with gravity, chemical cues, and multi-target memories
-
•
Following a catastrophe, ants quickly readjust the relative importance of cues
Wildlife Behavior; Biological Sciences; Entomology
Introduction
Navigation is a major component in the adaptive and ecological success of any animal species. Different environments demand different navigational strategies as they vary in their resource distribution, the sensory cues they offer, and their topological structure. The vast majority of current knowledge concerns navigation above ground, which heavily relies on visual cues and often takes place in environments, either two- or three-dimensional, that allow for relatively unconstrained motion. Life, however, also inhabits subterranean environments. Navigation in these dark constrained conditions (Tschinkel, 2005, Kimchi et al., 2004, Chittka et al., 1999) is far less understood.
Ants have attracted special attention in the study of navigation. Different ant species exhibit exceptional navigational skills despite an extremely small brain size (Wehner, 2003, Knaden and Graham, 2016). This has allowed for an extensive study of ant navigational strategies, of the mechanisms that underlie ant navigation, and of its ecological costs and benefits (Knaden and Graham, 2016, Wehner, 2003, Collett et al., 1998, Müller and Wehner, 1988, Merkle and Wehner, 2008). Similar to other species, ants depend on visual cues for navigation to a great extent (Merkle and Wehner, 2008), even when walking along pheromone trails (Czaczkes and Beckwith, 2018, Aron et al., 1993) or during nocturnal activity (Warrant and Dacke, 2011, Narendra et al., 2017). Correspondingly, the vast majority of research on ant navigation concerns movement on the surface of the ground. This stands at odds with the fact that ants spend a considerable fraction of their lives within their nests (Heyman et al., 2017).
The navigational capabilities that ants display above ground do not stop at the nest entrance: ants have preferred locations within the nest (Sendova-Franks and Franks, 1995, Mersch et al., 2013) to which they return repeatedly (Heyman et al., 2017). However, many of the navigation strategies that ants employ above the ground cannot be expected to carry over to intranidal navigation. Light does not penetrate underground. This renders the prevalent strategies of visual beaconing (Wehner et al., 1996, Graham et al., 2003, McLeman et al., 2002) and image matching (Lent et al., 2010) useless. Moreover, celestial bodies, often used as global positioning cues in various navigation mechanisms, are inaccessible. Here, we study the cues that are available underground and the ways in which ants integrate them into their navigational decisions.
What sources of navigational information are accessible inside the ant nest? Gravitational signals may account for an ant colony's organization along the vertical axis (Tschinkel, 1999, Tschinkel, 2003, Tschinkel, 2005, Tschinkel and Hanley, 2017), whereas magnetic sensation (Anderson and Vander Meer, 1993) could play a similar role in the horizontal direction. Chemical-encoded information is another possible source of navigational cues within the nest. Above ground such cues come in the form of pheromone trails (Holldobler and Wilson, 1990, David Morgan, 2009, Czaczkes et al., 2015), hydrocarbon gradients (Sturgis et al., 2011), and volatile chemical gradients (Steck et al., 2011, Buehlmann et al., 2012). The role of CO2 soil gradients in colony organization was studied within natural nests (Tschinkel, 2013). Recently, it was shown that chemical navigational cues within the nest allow the ants to distinguish between different nest chambers (Heyman et al., 2017).
Spatial memory may also be useful within the dark confines of the nest. An appealing mechanism in this respect is path integration, a prevalent navigational strategy that was studied mostly above ground but could potentially remain efficient under it (Kimchi et al., 2004) because ants were shown to perform path integration, which includes vertical components (Wohlgemuth et al., 2001). Another possible mechanism is motor learning, wherein movement sequences are memorized (Stamps, 1995, Srinivasan and Zhang, 2004). Ants were shown to apply motor learning while navigating in mazes with no visual landmarks (Macquart et al., 2008). Such self-referenced mechanisms reduce the dependence on external reference points, which may be unavailable within the nest (Collett and Collett, 2000, Wehner, 2003, Jeffery, 2003). However, independence from external references has its limitations: path integration must be accompanied by other navigational mechanisms to avoid runaway errors (Merkle et al., 2006, Merkle and Wehner, 2009, Müller and Wehner, 1988), whereas motor learning requires practicing the same route many times (Stamps, 1995).
Ants combine private and social cues in a variety of contexts (Cronin, 2013, Robinson et al., 2009, Czaczkes et al., 2011). Social information, which is formed by the combined knowledge of many individuals, is often reliable and stable (Galton, 1907) yet slow to respond to environmental changes (Feldman et al., 1996). In contrast, private information, which is based on individual learning, has shorter update times but is error-prone (Merkle et al., 2006, Merkle and Wehner, 2009, Müller and Wehner, 1988). The latter source of information becomes crucial in situations of rapid environmental changes where social information is either missing or misleading (Harrison et al., 1989). These two information sources therefore complement one another to allow for organized and adaptive behaviors (Rieucau and Giraldeau, 2011, Templeton and Giraldeau, 1995).
In this article, we use the brood-retrieval behavior of the species Camponotus fellah, to study how ants navigate their nest. We do this by tracking the trajectories of ants as they move from a misplaced brood pile outside the nest to a target chamber within the nest. We analyze which cues play important roles in the different parts of this trajectory. We find that, to navigate within the nest, the ants combine three independent sources of information. First are self-referenced cues where the ants memorize multiple target locations and orient toward them with no requirement for any visual or olfactory cues. Second are socially generated chemical cues that are placed at decision points located away from the destination and mark the route toward it. Third, we show that ant navigation is assisted by global gravitational cues. We go on to show how ants combine these different information sources and how individuals can adjust the weight attributed to conflicting cues in a way that allows them to adopt new routes while abandoning unrewarding ones. This fast individual learning process leads to global, stable improvement in the collective performance of the colony.
Results
Manipulating Nest Structure to Identify Relevant Navigational Cues
The ants' navigational capabilities were evaluated by following their performance in a brood-retrieval task. Experiments were initiated by placing a single pile of (≈50) brood items at a random location on the perimeter of the arena, outside the nest. Workers who encountered this misplaced brood tend to carry it into a nest chamber. To get from the arena to the nest chambers workers had to walk on the nest roof and climb down the entrance as the chamber section was embedded under the arena (see Methods section “Planar nest structure,” Supplemental Information section “Nest setup,” and Figure S1). To simulate the dark underground environment, the experimental setup was specifically designed to prevent the use of visual cues: all lights were in the infrared spectrum and nest corridors were sharply curved to block the line of sight. To verify that the ants carry the brood toward a designated goal within the nest we used an artificial nest that contains four symmetric corridors, three of which lead to identical chambers and one that leads to a dead end (see Figure 1A and Methods section “Planar nest structure”). Entries to the dead-end corridor by brood-carrying ants are defined as errors. We find that, in the absence of any manipulation, the proportion of errors in the brood retrieval task is extremely low . This establishes that brood-carrying ants do not randomly search for their destination chamber within the nest, but rather employ a reliable navigational strategy that takes them into specific corridors.
Figure 1.
Experimental Setup and Manipulations
(A) Nest structure scheme. The nest is composed of four identical corridors that lead to three identical chambers and one blocked chamber. The chamber unit is marked with blue stripes; the corridor unit is marked with yellow stripes; the entrance to the nest, which is included in the corridor unit, is marked by a small white circle; and the decision point is labeled with an asterisk. The corridors and chambers were covered by an infrared (IR) filter top (marked in pink). The chamber unit, the corridor unit, and the IR filter, which forms the center of the arena floor can rotate with respect to each other and to the foraging arena (marked by yellow, blue, and pink arrows; see Methods section “Planar nest structure,” Figure S1, and Video S1). The entire arena can also be rotated with respect to the laboratory frame of reference (marked by black arrow). Scale bar, 6.5 cm.
(B) Setup position under the different experimental manipulations and the resulting cue combinations. The blocked chamber is colored gray, and the corridor that originally led to it is white, whereas corridors that led to accessible chambers are colored yellow. Blue dots mark the possible presence of volatile chemicals emanating from accessible chambers into the entrance area. Compass rose signifies the spatial memory of the ants from the learning phase. The initial position of the setup is depicted in (B1). The four manipulations we employed are as follows. (B1) Control: the setup is rotated back and forth, retaining the original orientation of both corridor and chamber units, as well as the cue combinations. (B2) Chamber rotation: the chamber unit is rotated. (B3) Corridor rotation: the corridor unit is rotated. (B4) Full rotation: both the corridor and the chamber units are rotated, whereas their relative orientation is kept fixed. For more details refer to Figure S2.
(C) Timeline of the locations that an ant visits as she retrieves brood into the nest.
In our experimental setup, when a brood-carrying ant enters the nest, she immediately arrives at a decision point that is the junction between four corridors (Figure 1A, decision point is marked with an asterisk). Her decision to enter a specific corridor may be guided by one or several of the following cues: chemicals that are adsorbed to the surfaces of the corridor and sensed by direct tactile contact (tactile, solid at room temperature); volatile chemicals that diffuse away from a chamber (volatile, liquid or gas at room temperature); spatial memory, which reflects the ant's previous experience (spatial memory); and external cues such as the earth's gravitational or magnetic fields (global). To understand how the ants integrate these available cues toward reliable navigation (Wystrach et al., 2015, Wehner et al., 2016), we employed several confusion assays. We allowed the colony to return approximately half of the misplaced brood undisturbed (baseline phase) before applying one of several structural changes (test phase). These structural manipulations include independent rotations of one or more of the following parts of the setup: the chamber unit, the corridor unit (which includes the nest entrance), a large part of the arena floor, and the entire experimental setup (see Figure 1 and Video S1).
An ant retrieving brood before and directly after a corridor shift manipulation. The video was shot in a single take.
These rotations allowed us to isolate the effects of the aforementioned local cues (tactile, volatile, and spatial memory; Figure 1B, Supplemental Information section “Manipulation types and resulting cue combination,” and Figure S2) as well as of the global cues (such as external magnetic fields). Gravitational cues were studied separately by using a vertical setup as described in section “Navigation in Vertical Nests.”
To uncover the relevant cues and their relative importance we follow the complete trajectories of ants as they navigate from the misplaced brood to their destination within their nest. The structure of the Results section follows the timeline of this trip (see Figure 1C). Initially, an ant picks up a brood item from the misplaced brood and carries it to the nest entrance. After reaching the nest entrance, the ant approaches one of four identical corridors. Finally, the carrier ant enters one of the corridors and, eventually, the connected chamber where she places the brood.
From the Misplaced Brood Pile to the Nest Entrance
The earth's magnetic field or external air flows are examples of global cues that may assist ant orientation on her return trip to the nest. We tested the importance of global horizontal cues using the “arena rotation” manipulation, in which, halfway through the retrieval process, the entire experimental setup was rotated relative to the laboratory frame of reference (N = 4 experiments on two colonies). This manipulation maintains the links between the corridors, chambers, and any landmarks within the arena, but changes the orientation of these relative to the environment outside the setup. The spatial distribution of approaches relative to the arena frame of reference was unaffected by the manipulation (p = 2.1 × 10−3, N = 4 experiments, by the tail of the binomial distribution, see Figures 2A and 2B and Supplemental Information section “Arena rotation”). These results indicate that any horizontal directional cues that the ants may be using are confined to the experimental arena.
Figure 2.
Navigating from the Misplaced Brood to the Nest Entrance
(A) Histogram of the directions of initial approaches before the manipulation of the “arena rotation” experiments. Data is normalized by the number of retrievals. The direction pointing up is that of the blocked chamber before the rotation. Only the first corridor approach on the first retrieval of each ant per experimental phase is included. All experiments are pooled together.
(B) A similar histogram of the directions of initial approaches after the “arena rotation” manipulation.
(C) Trajectories of ants that are retrieving brood from the brood pile (marked white) to the nest entrance before (solid trajectories) and after (dashed trajectories) an “arena center rotation” manipulation that rotated the central part of the arena floor. Scale bar, 9 cm.
Arena-confined cues may come in the form of chemical cues, such as a pheromone trail that extends from the misplaced brood area through the nest entrance (Greene and Gordon, 2007, David Morgan, 2009, Czaczkes et al., 2015) and toward a specific direction within the nest. To test for the existence of such a trail, we rotated a large portion of the arena floor (“arena center rotation,” shaded area in Figure 2C) so that it lost its initial alignment with the misplaced brood area. This manipulation had no effect on the paths that ants followed on their way back to the nest (Figure 2C), ruling out the use of a pheromone trail on the external part of the route.
By means of elimination our results point toward two potential strategies by which the ants find their way from the misplaced brood pile and back to the nest entrance. These strategies are, indeed, well established for ant navigation outside the nest: following a gradient of volatile chemicals that may emanate from the nest (Buehlmann et al., 2012) and self-referenced spatial memory cues such as path integration (Collett and Collett, 2000, Wehner, 2003, Jeffery, 2003).
Preliminary Orientation within the Nest Relies on Spatial Memory
When ants first enter the nest they do not simply continue on the straight path they took from the brood pile to the nest entrance (contrary to Macquart et al., 2006, see Supplemental Information section “Approach direction has low correlation with entry angle” and Figure S3) but rather choose between one of four structurally identical corridors. At this point the ants cannot use the location of the external brood pile for orientation due to the dark conditions. Any deviation from random choice may rely on volatile chemicals that potentially emanate from inhabited chambers, tactile cues adhered to specific corridors, or spatial memory.
To study the relative importance of these cues, we analyzed the ants' response to rotational manipulations that either shifted the overall nest orientation or disrupted the connection between internal nest units (see Figure 1B). We find that the spatial distribution of initial approaches (relative to the laboratory frame of reference) before and after a manipulation is remarkably similar regardless of the type of manipulation (Figure 3A). As manipulations alter tactile and volatile cues, this result raises the possibility that ants use prior spatial information when deciding which corridor to approach. To test this, we divided the corridors, from all possible manipulations, into two groups by their orientation before the manipulation: those that were oriented in a direction that led to an accessible chamber and those that were oriented in a direction that led to a dead end (directions are taken with respect to the laboratory frame of reference, see Supplemental Information section “Manipulation types and resulting cue combination”). We find that the approach rate to the first group is significantly higher (Figure 3B, p = 1.2 × 10−2, z-test). Repeating a similar analysis for tactile and volatile cues (see Methods section “Rating cue importance”) did not yield any significant results implying that the ants do not rely on chemical cues when initially approaching a corridor. When considering combinations of cues, we also did not find a significant additive effect (see Supplemental information section “Additive effect of navigational cues”). This lack of dependence on environmental cues supports an assumption that the ants' initial direction of approach is guided by self-referential, idiothetic mechanisms.
Figure 3.
Preliminary Approach Guided by Spatial Memory
(A) Histograms of the directions of initial approaches (see also Figure S3) before (blue) and after (hashed purple) a manipulation for the four manipulation types, normalized to the total number of retrievals. The direction pointing up is that of the blocked chamber before the rotation. Only the first retrievals by ants that were outside the nest during the manipulations are included. All four experiment types are pooled together.
(B) Proportion of approaches to corridors carrying a positive cue, out of all approaches to corridors carrying either one or two positive cues. Only the first approach of the first retrieval of ants that were outside the nest during the manipulation (ants that have no knowledge of the postmanipulation nest structure) is included. * Indicates a proportion significantly different (p < 0.05) from the chance level of 0.5 (dotted line). Error bars signify SEMs.
(C) Distribution of angular difference between the direction of the corridor an ant left and the direction of the corridor she approached immediately afterward. Retrievals of ants who had just exited the blocked chamber, or the chamber across from the blocked chamber, were excluded. Approach directions are labeled as the direction of the “original” chamber that the ant had left, the direction directly “across” from this direction, the direction that leads to the “blocked” chamber, and the direction that leads to the accessible chamber that is placed “symmetrically” across from the blocked chamber. Expected random distribution is shown in orange (approaches divide equally between chambers symmetrically distant from the blocked chamber).
A well-established model of self-referential memory is path integration, which, in its most basic form, allows a navigator to calculate the distance and angle between its current position and an origin (Müller and Wehner, 1988). Under this model ants retrieving brood into the nest can be expected to return to the corridor through which they exited. To explore the possibility that the ants, indeed, apply basic path integration to approach a specific corridor we examined trajectories of brood-retrieving ants in non-manipulated nest structures. We find that on 52% (SEM = 1.8%, N = 763) of return trips, ants initially approach the corridor through which they exited the nest (Figure 3C). This is significantly higher than the 25% expected for a random approach direction (p < 10−50, by the tail of the binomial distribution). This observation is consistent with a basic path integration model with single target memory. However, the remaining 48% of retrieval trips that deviate and approach a different corridor from the one they exited (Figure 3C) are not symmetrically distributed around the target direction as one would expect if the ant applied basic path integration. We find that deviations toward the blocked chamber were significantly lower than deviations toward a symmetrically placed accessible chamber and, in fact, almost altogether absent (Figure 3C).
Ants Integrate Chemical Cues at Close Range
We next tested which cues are employed in an ant's decision to enter a corridor once she had approached it. We approximated the probability to enter an approached corridor by the measured ratio of entries to approaches and calculated this probability for every corridor in every experimental phase of the planar experiments depicted in Figure 1B. We averaged the resulting ratios across all corridors that share the same combination of cues (for example, positive tactile and volatile cues but not spatial memory). To rank the importance of the three cues (spatial memory, tactile, and volatile) we repeated the analysis used to create Figure 3B, as described in the previous section. We observed significantly increased probabilities to enter corridors in which the tactile markings are positive (Figure 4A, p < 1 × 10−20, z-test). Moreover, entry rates to corridors without tactile chemical markings was very low, at around 5%. These finding suggest that nest corridors are chemically marked and allow for indirect stigmergic (Theraulaz and Bonabeau, 1999) communication between the ants. These markings act as pointers that direct ant movement at the entrance to specific corridors within the nest.
Figure 4.
Entry by Tactile Chemical Cues
(A) Proportion of entry to approach rates to corridors carrying a positive cue (x-label), out of all entry to approach rates to corridors carrying either one or two positive cues. Only the first approach of the first retrieval is included. ∗ Indicates a proportion significantly different (p < 1 × 10−20) from the chance level of 0.5 (dotted line).
(B) Chemical analyses of corridor floors: for each experiment all four corridors were given a rank between one and four according to their chemical intensity (see Methods section “Chemical data analysis”). Corridors that were ranked 1 had the lowest chemical intensity, whereas corridors that ranked 4 had the highest. The distribution of ranks among accessible (dark blue) and blocked (light blue) corridors.
Error bars in both panels signify SEMs, see also Figure S4.
Hydrocarbon blends adhered to nest surfaces are known to regulate the spatial organization of ant colonies (Heyman et al., 2017). We therefore hypothesized that the tactile cues, which influence the ants' navigational choices, would be of the same nature and that the blocked corridor would display a distinct hydrocarbon profile. To test this assumption, we housed six C. fellah colonies in a Teflon replica of the artificial nest (Figure 1A) for 5 days and then extracted and analyzed the surface chemicals of different areas in the nest. In agreement with previous measurements we found that low-boiling hydrocarbons (“light,” chain length ≤ 21) were associated with entrance areas and corridors, whereas inner chambers had mostly high-boiling (“heavy,” chain length > 21) hydrocarbons (see Supplemental Information “Spatially resolved chemical profiling” and Figure S4). Corridors leading to accessible chambers are higher in heavy hydrocarbons, whereas blocked corridors are generally lower (see Figure 4B and Methods section “Chemical data analysis,” p = 2.57 × 10−2, N = 24 samples, by the tail of the binomial distribution); this implies that blocked corridors are indeed chemically distinguishable from other routes.
To summarize, our results suggest that within the nest ants follow a two-stage decision process: spatial-memory-based navigation is applied when choosing a general direction of approach; later, when the ant is close enough to sense tactile cues that are adsorbed to the nest's surfaces, these are integrated into the decision (see Video S1).
Individual Learning Leads to Global Short-Term Improvement in Colony Performance
Ant colonies depend on their ability to adapt to an ever-changing environment (Dussutour et al., 2009, Gordon, 2002, Reid et al., 2011). We examined whether colonies can adapt their brood-retrieval paths to changes in nest structure within the course of a single experiment (approximately 1 h).
To facilitate the detection of the effects of learning, we designed modified manipulations that were aimed to induce a catastrophe in the nest structure by dissociating the connection between different navigational cues and their meaning (see Methods section “Learning experiments”). These manipulations led to an error rate of 8% (over all entries after the manipulation, SEM = 0.009).
In each experiment, we grouped all post-manipulation retrieval events (N) into two chronological equal-sized bins (N/2, the two bins contained equal number of events). We then calculated the global failure rate for every bin in every experiment. We defined failure rate as the percentage of retrievals to the blocked corridor out of the total number of retrievals. We find that the failure rate was significantly reduced between bins; this implies a global improvement in colony performance over time (Figure 5A). The results presented thus far show that ants use both personal knowledge in the form of spatial memory and social information in the form of tactile chemicals to navigate within their nest. The global improvement could, therefore, be the outcome of either collective or individual learning. Individual learning implies that experienced individuals have gradually adjusted their own navigational strategy in the new nest structure. Collective learning allows ants to improve in a manner that is independent of their personal experience and could result, for example, from an accumulation of scent marks. These mechanisms are not mutually exclusive, and we tested for each of them independently.
Figure 5.
Individual Learning Leads to Global Short-Term Improvement of the Colony's Performance
All error rates refer to retrievals after the manipulation.
(A) The proportion of ants that enter the corridor that leads to a blocked chamber (failure rate) in two successive phases. ∗ Indicates significantly different proportions (p < 0.05).
(B) Similar failure rates as calculated using a subset of the full data, which is restricted to retrievals by ants that participated in only one of the two successive phases.
(C) Mean failure rate of ants that participated in over three retrievals after the manipulation. Here first versus last retrievals are defined per ant and not per the entire colony as in (A and B). ∗ Indicates significantly differentrates (p < 0.05).
(D) Examination dynamics of corridors that display tactile chemicals. Previous memory (blue) refers to dead-end corridors positioned in a direction that led to an accessible chamber before a manipulation (dead-end corridors that are associated with positive spatial memories). No memory (red) refers to dead-end corridors positioned in a direction that led to the blocked chamber before the manipulation (dead-end corridors that are associated with negative spatial memories). New memory (yellow) refers to fully open corridors positioned in a direction that led to the blocked chamber before the manipulation (fully open corridors that are associated with negative spatial memories). Data for the first retrievals (three leftmost bins) include only “uninformed” ants that were outside the nest during the manipulation and could not obtain updated structural information of the nest. The three rightmost bins refer to non-first retrievals by all ants. The ants in this dataset are considered “informed” as they have occupied the nest after the manipulation. Error bars in all panels signify SEMs.
To test for collective learning in the form of accumulation of scent marks, we filtered the binned data such that it contained retrievals by ants that participated in only one of the binned phases. This ensures that the experience distributions of ants in the two phases are similar and cancel the effect of individual learning. If global improvement is the result of collective learning, we expect ants that participated in the second phase to display a lower error rate compared with those that participated in the first phase, owing to the accumulation of a social navigational cue. The failure rate of the filtered bins is almost identical (Figure 5B, chi-square test for independence, χ2(1,N = 78)<0.01, p = 0.98), ruling out collective learning on the timescale of this experiment.
To determine whether global short-term improvement in colony performance stems from individual learning, we filtered the data such that it contained only ants that participated in at least four retrieval trips and had at least one error (N = 41 ants). For each selected individual in each experiment, we divided the total number of retrievals into two bins and calculated the two individual failure rates. We find that the failure rate is significantly higher in the first bin than in the second one (Figure 5C, Wilcoxon signed-rank test, w = 310, p < 0.025, n = 41). This implies that, on the short timescale following a manipulation (≈1 h), the global improvement in colony performance results from individuals that independently react to the change and dynamically adjust their navigation strategy.
Further support for individual learning comes when considering examination dynamics of corridors that display tactile chemicals but differ in their other cues (Figure 5D). In the initial examination each ant tends to approach corridors that are located in a direction that once led to an accessible chamber, not knowing that it now leads to a dead end (previous memory). In subsequent retrievals the examination rate of such corridors decreases. Accordingly, examination rates of directions that led to the blocked chamber before the manipulation and to an accessible chamber after it (new memory) display the opposite trend (Figure 5D). Examination rates of directions that led to the blocked chamber both before and after the manipulation (no memory) remained unchanged.
Navigation in Vertical Nests
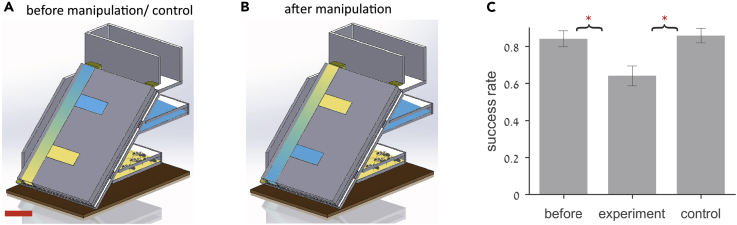
Ant nests, including the nests of many species in the Camponotus genus, significantly extend in the vertical direction (Tschinkel, 2005); in such nests the earth gravitational pull may serve as an important global orientation cue. To test how ants utilize gravitational cues during intranidal navigation, we constructed an artificial nest that consists of two identical horizontal chambers connected through horizontal corridors to a 45∘ angle shaft that leads to the nest entrance (Figures 6A and 6B). In the first set of experiments (N = 5) we introduced a colony into this structure and observed the distribution of ants across the two nest chambers. We found that the ants exclusively housed all brood items in the lower chambers (N = 5 experiments, p = 0.0313, by the tail of the binomial distribution). This cannot be explained by a general preference to place brood far from the entrance because in artificial horizontal nests the location of the brood chamber does not correlate with the distance from the nest entrance (Heyman et al., 2017). Taken together these observations support the use of gravity as a navigational cue.
Figure 6.
Global Orientation Cues
(A) A diagram of the vertical nest. Before any manipulation most of the ants together with the queen and the brood are found in the bottom chamber (blue). The corridor that leads to this chamber is labeled in blue. The top chamber and the corridor that leads to it are labeled yellow. The directionality of the vertical corridor is indicated by a color gradient. Scale bar, 5 cm.
(B) Illustration of the post-manipulation setup: after the ants return a significant portion of the brood the horizontal corridors are switched and the vertical corridor flipped.
(C) Success rate, defined as the fraction of brood items retrieved to the bottom chamber, before (left bar) and after a flip manipulation (center bar) and a control manipulation (right bar). *p < 0.05. Error bars signify SEMs.
A second set of experiments (N = 6) was designed to test the role of tactile cues in a vertical scenario. We introduced a colony into an identical nest structure for a habituation period of 7 days during which the ants were allowed to move freely inside the structure permitting any natural accumulation of chemicals (Heyman et al., 2017). Following habituation a measurement was initiated by placing 40 brood items in the foraging arena. As with previous experiments, the ants were allowed to return approximately half of the brood before one of two structural manipulations were performed: (1) flip—the main shaft was vertically flipped and the connecting horizontal corridors switched; (2) control—the main shaft and the corridors were removed from the nest structure and then returned to their previous locations (see Figure 6B for schematic illustration). Flip experiments are designed to create a discrepancy between tactile, chemical cues on the corridor surfaces and gravitational cues. In both types of experiments, ants either placed brood items in the main corridor or brought them to one of the chambers. Before the manipulation, in both control and flip experiments (N = 3 of each), a majority brood items were transferred into the bottom chamber (mean value of 84%, Figure 6C). After the manipulation, these percentages dropped to 64% (52 of N = 81 total retrievals, p < 10−15, by the tail of the binomial distribution) for the flip experiments but remained constant for the control experiment (86%, 67 of N = 78 retrievals, Figure 6C). Unlike the planar experiments, the vertical nest design did not allow us to break down the ants' trajectory to an initial approach that is followed by an actual entry. This is because the structure of the vertical nest constrains the ants to pass by the top corridor on their way to the bottom one.
The structure of the vertical nest did, however, allow us to assess the reduction in navigation efficiency due to a mismatch between personal information, in the form of gravity and spatial memory, and social information, in the form of tactile chemicals. We compared retrieval times of flip and control experiment before and after the manipulation (see Methods section “Vertical Setup”). We find that in flip experiments retrieval events were, on average, significantly longer after the manipulation (13.49 ± 3.19 s in the before phase compared with 20.9 ± 3.3 s in the after phase). Control experiments showed no such effect (13.35 ± 4.61 s in the before phase compared with 11.66 ± 1.73 s in the after phase). These results indicate that inside the nest gravitational pull does not override tactile cues. A possible navigational scheme could be that the ants are guided to the general direction of their destination by the gravitational pull, and locate the precise branch into which they turn using tactile cues.
Discussion
Ant nests and open-air environments differ in the type and accessibility of the navigational reference points they supply. Above ground, visual stimuli provide an abundance of long-range cues, which stand at the base of most known navigational strategies (Merkle and Wehner, 2008, Hölldobler, 1980, Levy, 2001, Müller and Wehner, 2007, Graham and Cheng, 2009). Long-range cues provide valuable orientation information such as an absolute compass (Wehner and Muller, 2006), beacons (Merkle and Wehner, 2008, Lent et al., 2010), or learned scenes (Wystrach et al., 2011) by which an animal may continuously adjust its trajectory toward the target. The dependence on long-range cues is so high that even nocturnal insects have evolved the ability to recognize landmarks, discern colors, and use celestial cues with very little light (Warrant and Dacke, 2011, Narendra et al., 2017). The situation in underground environments is very different. First, such environments are naturally devoid of any visual cues. Second, motion through constrained underground environments does not allow for continuous adjustments but, rather, entails corrections at specific junction points. In this article, we studied how ants confront the challenge of intranidal navigation.
Although an ant nest is relatively poor in long-range cues, some may still be available. We found no evidence for volatile chemical beacons that diffuse through the nest to mark the direction to a target chamber (Figures 3 and 4). This may be the result of the difficulty to maintain time-stable chemical gradients in the poorly ventilated atmosphere of the nest. We further found no evidence that this species uses the earth's magnetic compass for orientation. The only long-range cue identified is that set by gravity (Figure 6). However, simple discrimination between up and down cannot be sufficient for navigating through the intricate three-dimensional structure of an ant nest. To overcome this lack of long-range cues, ant navigation utilizes local cues in the form of self-produced, social chemicals that are adsorbed to nest surfaces (Figure 4). Contrary to volatile chemicals, these chemical pointers are located at specific points in the nest such that long-term informative patterns are easier to maintain. Note that these chemical cues occur in locations that are spatially distant from the target destination. This is reminiscent of pheromone trail behavior evident above the surface of the ground. Further work will be required to test whether these chemical pointers are indeed part of pheromone trails that extend across the nest. Together with the finding that ants use chemicals to differentially mark different nest chambers according to their function (Heyman et al., 2017) this suggests the possibility that the nest may contain several overlapping pheromone trail networks that allow ants of different task groups to reach their specific underground destinations.
The lack of long-range information, either visual or olfactory, entails a larger reliance on memory and self-referenced orientation. Indeed, the ants' preliminary approach within the nest is completely set by their internal directional memories regarding the locations of the available chambers (Figures 2A, 2B, and 3A–3C). The fact that ants refrained from approaching the blocked corridor but did approach all other corridors (Figure 3C) implies that they memorized several targets (or combinations of targets and non-targets such as the blocked chamber) (Schatz et al., 1999). One self-referenced navigation model that may account for this is motor learning in which the animal performs a memorized sequence of movements to make its way between two familiar places (Macquart et al., 2008). An extension of motor learning to multiple destinations is an appealing model as it reduces the need for cognitive computations along the trip by using procedural routines. On the other hand, this model assumes that the animal has some practice or a priori spatial knowledge (Stamps, 1995). Such knowledge is, to a large degree, inaccessible in our experimental design in which brood items are introduced immediately before the experiment starts. Furthermore, if they were indeed employing motor learning, we would expect the trajectories of different trips by the same individual to be almost identical. This is not the case as apparent in Figures 2C and 3C. Another model that is widely supported by navigation above ground is path integration. In the most basic model of path integration, an animal internally stores a single homing vector (Müller and Wehner, 1988) often directed toward the point at which the current trip was initiated. In this case, approaches to multiple chambers may be attributed to random noise, which is, indeed, to be expected in the dark nest, where external references are not available (Merkle et al., 2006, Merkle and Wehner, 2009, Müller and Wehner, 1988). Yet, the non-symmetric distribution of ant approach directions (Figure 3C) does not support this hypothesis. Therefore these two simpler models cannot provide an explanation of our experimental results. Our findings are, however, compatible with modern versions of insect navigation theory that permit multi-target memories (Cruse and Wehner, 2011, Menzel et al., 2005).
Our findings suggest that ants combine publicly available cues and privately held spatial memory to navigate inside the nest. The ants first approach the general area of their destination by spatial memory and then locate the precise path using tactile social cues. It remains to be tested whether ants also communicate with each other and use direct social interactions for intranidal navigational purposes. It is often the case that social animals favor personally held over socially transmitted information (Grüter and Ratnieks, 2011, Webster and Laland, 2008). Social information is prone to noise (Razin et al., 2013) and may become outdated (Laland and Williams, 1998) as it spreads between individuals. For example, ants that travel along pheromone trails were shown to favor private information, which contains more details over social information, which tends to be ambiguous (Czaczkes and Beckwith, 2018). This hierarchy may break down when private information becomes unreliable (Fonio et al., 2016). Accordingly, our results indicate that, in the information-poor environment of the nest, ants tend to favor social signals and rarely enter an unmarked corridor. Interestingly, following catastrophic changes to nest structure that dissociate social cues from their original meaning, ants quickly readjust (see also Dupuy et al., 2006). They rapidly learn to attribute more weight to private knowledge sometimes even after a single trip (Figure 5). In the long run, individual adjustments made by multiple ants result in changes to the chemical signatures within the different nest corridors. This chemical remarking of the nest, a form of collective learning on a longer timescale, ultimately relieves the conflict between the social and private information.
The environment in which the animal navigates dictates the nature of the available reference points and hence the navigation strategy. Above the surface, ants navigate large distances and use long-range visual cues as references and their reliance on idiothetic cues is dependent on the existence of such external references. Underground, ants do not use a completely disjoint navigational toolbox. Nevertheless, the unique conditions underground and the resulting differences in cue reliability lead to modified priorities in the ants' navigational strategies. This leads to careful integration of short-range cues present at crucial decision points and privately held spatial memories encompassing multiple destinations. The differences between these two navigation tactics could, in the future, contribute to our understanding of the neurocomputational aspects of insect navigation.
Limitations of Study
In this work, we studied the mechanisms that ants use to navigate within their nests. The measurements required for this study include single ant tracking and chamber surface chemical composition and are, to date, impossible to achieve in the field. Therefore this study was performed in artificial laboratory nests. Although the nests were constructed to weakly mimic the natural structure of the nest (chambers, and corridors, vertical and horizontal components) they are far from being natural. Therefore these results should be understood as a first glimpse into ant subterranean navigation and the mechanisms that are involved rather than a comprehensive answer to this aspect of ant navigation. Furthermore, the nest structures used in this study were relatively simple with a small number of junctions and chambers. Future studies using multiple sequential decision points may provide a wider view on ant in nest navigation and allow us to test our hypothesis that the ant nest is marked by overlapping trail networks each leading to different functional destinations.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We would like to thank Antoine Wystrach for critical revision of the manuscript and Abraham Hefetz for useful discussions; Benjamin Sharon, Guy Han, and Gershon Elazar for technical help; and Lior Baltiansky and Netta Reshef for experimental aid. This research was supported by Israel Science Foundation (ISF) grant 833/15. Support given by the Heineman Foundation through Minerva. O.F. was supported by the ongoing generosity of the Clore Foundation.
Author Contributions
Y.H and Y.V. designed the experiments, conducted the experiments, analyzed the data, and wrote the paper. O.F designed the study and the project, obtained funding, supervised the study, and wrote the paper.
Declaration of Interests
The authors declare no competing interests.
Published: April 26, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2019.04.003.
Supplemental Information
References
- Anderson J.B., Vander Meer R.K. Magnetic orientation in the fire ant, Solenopsis invicta. Naturwissenschaften. 1993;80:568–570. [Google Scholar]
- Aron S., Beckers R., Deneubourg J.L., Pasteels J.M. Memory and chemical communication in the orientation of two massrecruiting ant species. Insectes Sociaux. 1993;40:369–380. [Google Scholar]
- Buehlmann C., Hansson B.S., Knaden M. Path integration controls nestplume following in desert ants. Curr. Biol. 2012;22:645–649. doi: 10.1016/j.cub.2012.02.029. [DOI] [PubMed] [Google Scholar]
- Chittka L., Williams N.M., Rasmussen H., Thomson J.D. Navigation without vision: bumblebee orientation in complete darkness. Proc. Biol. Sci. 1999;266:45–50. [Google Scholar]
- Collett M., Collett T.S., Bisch S., Wehner R. Local and global vectors in desert ant navigation. Nature. 1998;394:269–272. [Google Scholar]
- Collett T.S., Collett M. Path integration in insects. Curr. Opin. Neurobiol. 2000;10:757–762. doi: 10.1016/s0959-4388(00)00150-1. [DOI] [PubMed] [Google Scholar]
- Cronin A.L. Conditional use of social and private information guides house-hunting ants. PLoS One. 2013;8:e64668. doi: 10.1371/journal.pone.0064668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruse H., Wehner R. No need for a cognitive map: decentralized memory for insect navigation. PLoS Comput. Biol. 2011;72:e1002009. doi: 10.1371/journal.pcbi.1002009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czaczkes T.J., Beckwith J.J. Information synergy: adding unambiguous quality information rescues social information use in ants. Biorxiv. 2018:219980. [Google Scholar]
- Czaczkes T.J., Grüter C., Jones S.M., Ratnieks F.L. Synergy between social and private information increases foraging efficiency in ants. Biol. Lett. 2011;7:521–524. doi: 10.1098/rsbl.2011.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czaczkes T.J., Grüter C., Ratnieks F.L. Trail pheromones: an integrative view of their role in social insect colony organization. Annu. Rev. Entomol. 2015;60:581–599. doi: 10.1146/annurev-ento-010814-020627. [DOI] [PubMed] [Google Scholar]
- David Morgan E. Trail pheromones of ants. Physiol. Entomol. 2009;34:1–17. [Google Scholar]
- Dupuy F., Sandoz J.C., Giurfa M., Josens R. Individual olfactory learning in Camponotus ants. Anim. Behav. 2006;72:1081–1091. [Google Scholar]
- Dussutour A., Beekman M., Nicolis S.C., Meyer B. Noise improves collective decision-making by ants in dynamic environments. Proc. Biol. Sci. 2009;276:4353–4361. doi: 10.1098/rspb.2009.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman M.W., Aoki K., Kumm J. Individual versus social learning: evolutionary analysis in a fluctuating environment. Anthropol. Sci. 1996;104:209231. [Google Scholar]
- Fonio E., Heyman Y., Boczkowski L., Gelblum A., Kosowski A., Korman A., Feinerman O. A locally-blazed ant trail achieves efficient collective navigation despite limited information. Elife. 2016;5:e20185. doi: 10.7554/eLife.20185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galton F. Vox populi (The wisdom of crowds). Nature. 1907;75:450–451. [Google Scholar]
- Gordon D.M. The regulation of foraging activity in red harvester ant colonies. Am. Nat. 2002;159:509–518. doi: 10.1086/339461. [DOI] [PubMed] [Google Scholar]
- Graham P., Cheng K. Ants use the panoramic skyline as a visual cue during navigation. Curr. Biol. 2009;19:R935–R937. doi: 10.1016/j.cub.2009.08.015. [DOI] [PubMed] [Google Scholar]
- Graham P., Fauria K., Collett T.S. The influence of beacon-aiming on the routes of wood ants. J. Exp. Biol. 2003;206(Pt 3):535–541. doi: 10.1242/jeb.00115. [DOI] [PubMed] [Google Scholar]
- Greene M.J., Gordon D.M. How patrollers set foraging direction in harvester ants. Am. Nat. 2007;170:943–948. doi: 10.1086/522843. [DOI] [PubMed] [Google Scholar]
- Grüter C., Ratnieks F.L.W. Honeybee foragers increase the use of waggle dance information when private information becomes unrewarding. Anim. Behav. 2011;81:949–954. [Google Scholar]
- Harrison J.F., Fewell J.H., Stiller T.M., Breed M.D. Effects of experience on use of orientation cues in the giant tropical ant. Anim. Behav. 1989;37:869–871. [Google Scholar]
- Heyman Y., Shental N., Brandis A., Hefetz A., Feinerman O. Ants regulate colony spatial organization using multiple chemical road-signs. Nat. Commun. 2017;8:15414. doi: 10.1038/ncomms15414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölldobler B. Canopy orientation: a new kind of orientation in ants. Science. 1980;210:86–88. doi: 10.1126/science.210.4465.86. [DOI] [PubMed] [Google Scholar]
- Holldobler B., Wilson E.O. Harvard University Press; 1990. The Ants; p. 732. [Google Scholar]
- Jeffery K.J. Oxford University Press Oxford; 2003. The Neurobiology of Spatial Behaviour. [Google Scholar]
- Kimchi T., Etienne A.S., Terkel J. A subterranean mammal uses the magnetic compass for path integration. Proc. Natl. Acad. Sci. U S A. 2004;101:1105–1109. doi: 10.1073/pnas.0307560100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaden M., Graham P. The sensory ecology of ant navigation: from natural environments to neural mechanisms. Annu. Rev. Entomol. 2016;61:63–76. doi: 10.1146/annurev-ento-010715-023703. http//www.annualreviews.org/doi/10.1146/annurev-ento-010715-023703 [DOI] [PubMed] [Google Scholar]
- Laland K.N., Williams K. Social transmission of maladaptive information in the guppy. Behav. Ecol. 1998;9:493–499. [Google Scholar]
- Lent D.D., Graham P., Collett T.S. Image-matching during ant navigation occurs through saccade-like body turns controlled by learned visual features. Proc. Natl. Acad. Sci. U S A. 2010;107:16348–16353. doi: 10.1073/pnas.1006021107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy R.J. Oceili: a celestial compass in the desert ant cataglyphis. Science. 2001;429:192–194. doi: 10.1126/science.228.4696.192. [DOI] [PubMed] [Google Scholar]
- Macquart D., Garnier L., Combe M., Beugnon G. Ant navigation en route to the goal: signature routes facilitate way-finding of Gigantiops destructor. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2006;192:221–234. doi: 10.1007/s00359-005-0064-7. [DOI] [PubMed] [Google Scholar]
- Macquart D., Latil G., Beugnon G. Sensorimotor sequence learning in the ant Gigantiops destructor. Anim. Behav. 2008;75:1693–1701. [Google Scholar]
- McLeman M.A., Pratt S.C., Franks N.R. Navigation using visual landmarks by the ant Leptothorax albipennis. Insectes Sociaux. 2002;49:203–208. [Google Scholar]
- Menzel R., Greggers U., Smith A., Berger S., Brandt R., Brunke S., Bundrock G., Hülse S., Plümpe T., Schaupp F. Honey bees navigate according to a map-like spatial memory. Proc. Natl. Acad. Sci. U S A. 2005;102:3040–3045. doi: 10.1073/pnas.0408550102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkle T., Knaden M., Wehner R. Uncertainty about nest position influences systematic search strategies in desert ants. J. Exp. Biol. 2006;209:3545–3549. doi: 10.1242/jeb.02395. [DOI] [PubMed] [Google Scholar]
- Merkle T., Wehner R. Landmark guidance and vector navigation in outbound desert ants. J. Exp. Biol. 2008;211:3370–3377. doi: 10.1242/jeb.022715. [DOI] [PubMed] [Google Scholar]
- Merkle T., Wehner R. Repeated training does not improve the path integrator in desert ants. Behav. Ecol. Sociobiol. 2009;63:391. [Google Scholar]
- Mersch D.P., Crespi A., Keller L. Tracking individuals shows spatial fidelity is a key regulator of ant social organization. Science. 2013;340:1090–1093. doi: 10.1126/science.1234316. [DOI] [PubMed] [Google Scholar]
- Müller M., Wehner R. Wind and sky as compass cues in desert ant navigation. Naturwissenschaften. 2007;94:589–594. doi: 10.1007/s00114-007-0232-4. [DOI] [PubMed] [Google Scholar]
- Müller M., Wehner R. Path integration in desert ants, Cataglyphis fortis. Proc. Natl. Acad. Sci. U S A. 1988;85:5287–5290. doi: 10.1073/pnas.85.14.5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendra A., Kamhi J.F., Ogawa Y. Moving in dim light: behavioral and visual adaptations in nocturnal ants. Integr. Comp. Biol. 2017;57:11041116. doi: 10.1093/icb/icx096. [DOI] [PubMed] [Google Scholar]
- Razin N., Eckmann J.P., Feinerman O. Desert ants achieve reliable recruitment across noisy interactions. J. R. Soc. Interface. 2013;10:20130079. doi: 10.1098/rsif.2013.0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid C.R., Sumpter D.J., Beekman M. Optimisation in a natural system: argentine ants solve the towers of Hanoi. J. Exp. Biol. 2011;214:50–58. doi: 10.1242/jeb.048173. [DOI] [PubMed] [Google Scholar]
- Rieucau G., Giraldeau L.A. Exploring the costs and benefits of social information use: an appraisal of current experimental evidence. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2011;366:949–957. doi: 10.1098/rstb.2010.0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson E.J.H., Richardson T.O., Sendova-Franks A.B., Feinerman O., Franks N.R. Radio tagging reveals the roles of corpulence, experience and social information in ant decision making. Behav. Ecol. Sociobiol. 2009;63:627–636. [Google Scholar]
- Schatz B., Lachaud J.P., Beugnon G. Spatio-temporal learning by the ant Ectatomma ruidum. J. Exp. Biol. 1999;202:1897–1907. doi: 10.1242/jeb.202.14.1897. [DOI] [PubMed] [Google Scholar]
- Sendova-Franks A.B., Franks N.R. Spatial relationships within nests of the antLeptothorax unifasciatus (Latr.) and their implications for the division of labour. Anim. Behav. 1995;50:121–136. [Google Scholar]
- Srinivasan M.V., Zhang S. Visual motor computations in insects. Annu. Rev. Neurosci. 2004;27:679–696. doi: 10.1146/annurev.neuro.27.070203.144343. [DOI] [PubMed] [Google Scholar]
- Stamps J. Motor learning and the value of familiar space. Am. Nat. 1995;146:41–58. [Google Scholar]
- Steck K., Hansson B.S., Knaden M. Desert ants benefit from combining visual and olfactory landmarks. J. Exp. Biol. 2011;214:1307–1312. doi: 10.1242/jeb.053579. [DOI] [PubMed] [Google Scholar]
- Sturgis S.J., Greene M.J., Gordon D.M. Hydrocarbons on harvester ant (Pogonomyrmex barbatus) middens guide foragers to the nest. J. Chem. Ecol. 2011;37:514–524. doi: 10.1007/s10886-011-9947-y. [DOI] [PubMed] [Google Scholar]
- Templeton J.J., Giraldeau L.A. Patch assessment in foraging flocks of European starlings: evidence for the use of public information. Behav. Ecol. 1995;6:65–72. [Google Scholar]
- Theraulaz G., Bonabeau E. A brief history of stigmergy. Artif. Life. 1999;5:97–116. doi: 10.1162/106454699568700. [DOI] [PubMed] [Google Scholar]
- Tschinkel W. The nest architecture of the ant, Camponotus socius. J. Insect Sci. 2005;5:1–18. doi: 10.1093/jis/5.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschinkel W.R. Sociometry and sociogenesis of colonies of the harvester ant, Pogonomyrmex badius: distribution of workers, brood and seeds within the nest in relation to colony size and season. Ecol. Entomol. 1999;24:222–237. [Google Scholar]
- Tschinkel W.R. Subterranean ant nests: trace fossils past and future? Palaeogeogr. Palaeoclimatol. Palaeoecol. 2003;192:321–333. [Google Scholar]
- Tschinkel W.R., Hanley N. Vertical organization of the division of labor within nests of the Florida harvester ant, Pogonomyrmex badius. PLoS One. 2017;12:e0188630. doi: 10.1371/journal.pone.0188630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschinkel W.R. Florida harvester ant nest architecture, nest relocation and soil carbon dioxide gradients. PLoS One. 2013;8:e59911. doi: 10.1371/journal.pone.0059911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrant E., Dacke M. Vision and visual navigation in nocturnal insects. Annu. Rev. Entomol. 2011;56:239–254. doi: 10.1146/annurev-ento-120709-144852. [DOI] [PubMed] [Google Scholar]
- Webster M.M., Laland K.N. Social learning strategies and predation risk: minnows copy only when using private information would be costly. Proc. Biol. Sci. 2008;275:2869–2876. doi: 10.1098/rspb.2008.0817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehner R. Desert ant navigation: how miniature brains solve complex tasks. J. Comp. Physiol. A. Neuroethol. Sens. Neural Behav. Physiol. 2003;189:579–588. doi: 10.1007/s00359-003-0431-1. [DOI] [PubMed] [Google Scholar]
- Wehner R., Hoinville T., Cruse H., Cheng K. Steering intermediate courses: desert ants combine information from various navigational routines. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2016;202:459–472. doi: 10.1007/s00359-016-1094-z. [DOI] [PubMed] [Google Scholar]
- Wehner R., Michel B., Antonsen P. Visual navigation in insects: coupling of egocentric and geocentric information. J. Exp. Biol. 1996;199:129140. doi: 10.1242/jeb.199.1.129. [DOI] [PubMed] [Google Scholar]
- Wehner R., Muller M. The significance of direct sunlight and polarized skylight in the ant's celestial system of navigation. Proc. Natl. Acad. Sci. U S A. 2006;103:12575–12579. doi: 10.1073/pnas.0604430103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlgemuth S., Ronacher B., Wehner R. Ant odometry in the third dimension. Nature. 2001;411:795–798. doi: 10.1038/35081069. [DOI] [PubMed] [Google Scholar]
- Wystrach A., Beugnon G., Cheng K. Landmarks or panoramas: what do navigating ants attend to for guidance? Front. Zool. 2011;8:21. doi: 10.1186/1742-9994-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wystrach A., Mangan M., Webb B. Optimal cue integration in ants. Proc. Biol. Sci. 2015;282:20151484. doi: 10.1098/rspb.2015.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
An ant retrieving brood before and directly after a corridor shift manipulation. The video was shot in a single take.