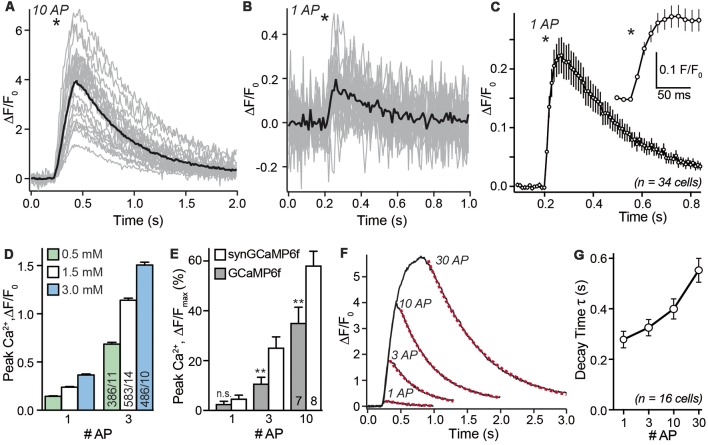
Figure 3.
Stimulus-response relationship of synGCaMP6f-measured Ca2+ transients. (A) Stimulation with a train of 10 AP (50 Hz) reliably induced a transient increase in synGCaMP6f fluorescence that varied in size. The sample recording evaluated 23 ROIs of presynaptic boutons from a single neuron (gray lines). Black line shows averaged response; asterisk indicates onset of the stimulation. Panel (B) in the same ROIs shown in (A) a single AP elicited smaller calcium transients. Black line, averaged response to one AP. (C) Combining the averaged responses of 34 neurons, representing 887 ROIs, reveals the kinetics of fluorescence changes in response to a single AP. The circles illustrate the fluorescence of a 10 ms exposure period (recording with 100 Hz) averaged from 34 separate recordings. The maximum of the synGCaMP6f fluorescence intensity is sampled 60–70 ms after the AP and it lasts more than 0.8 s to reach the baseline level again. Inset shows the initial response at higher resolution. Asterisks indicates onset of stimulation. (D) Recording of presynaptic calcium transients induced by one or three APs in different concentrations of extracellular calcium [(Ca2+)e]. In normal recordings, 1.5 mM [(Ca2+)e] was used. (E) Peak values of Ca2+ transients in response to increasing numbers of APs are compared between GCaMP6f and synGCaMP6f and normalized to Fmax as revealed with ionomycin application at the end of each experiment. These measurements indicate stronger fluorescence changes with the synaptophysin-coupled GCaMP. (F) Averaged responses (compare black lines in A,B) from 32 presynaptic boutons of a single neuron to 1, 3, 10 and 30 APs that underwent a mono-exponential decay time fit (dotted red line). (G) Analysis of decay time in >500 presynaptic ROIs from 16 neurons reveals a moderate increase to longer AP trains. Data in (C–E,G) are mean ± SEM; in (E), columns with equal stimulation are compared by student’s t-test; n.s.: p > 0.05; **p < 0.01.