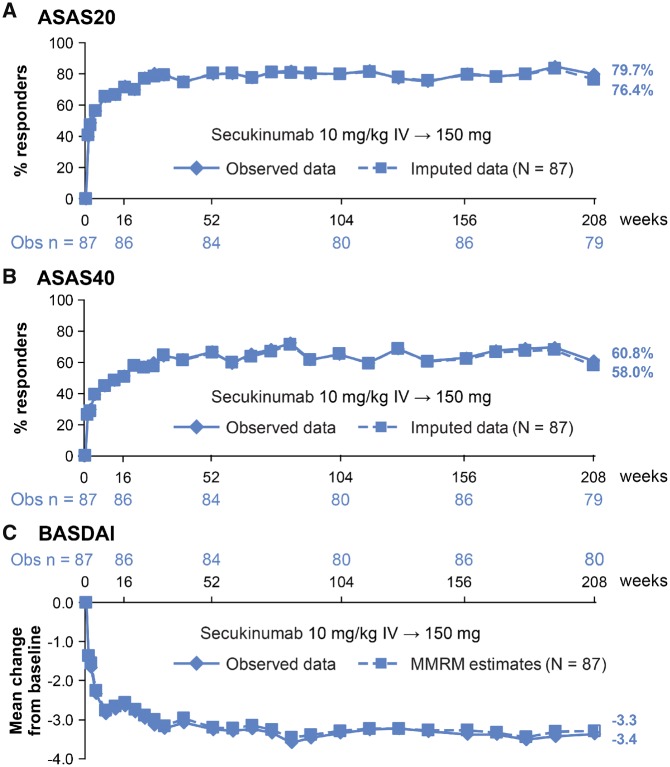
Fig. 1.
(A) ASAS20, (B) ASAS40 responses and (C) mean change from baseline in BASDAI through Wk208
Data are presented for patients originally randomized to secukinumab IV-150 mg. ASAS 20/40 are reported as observed and using multiple imputation. For BASDAI, mean change from baseline is reported as observed data and as least-square mean change (MMRM analysis). ASAS: Assessment of Spondyloarthritis International Society; IV: intravenous; MMRM: mixed model repeated measures; n: number of evaluable patients; N: total number of patients initially randomized to secukinumab 150 mg at baseline; Obs: observed data; Wk: week.