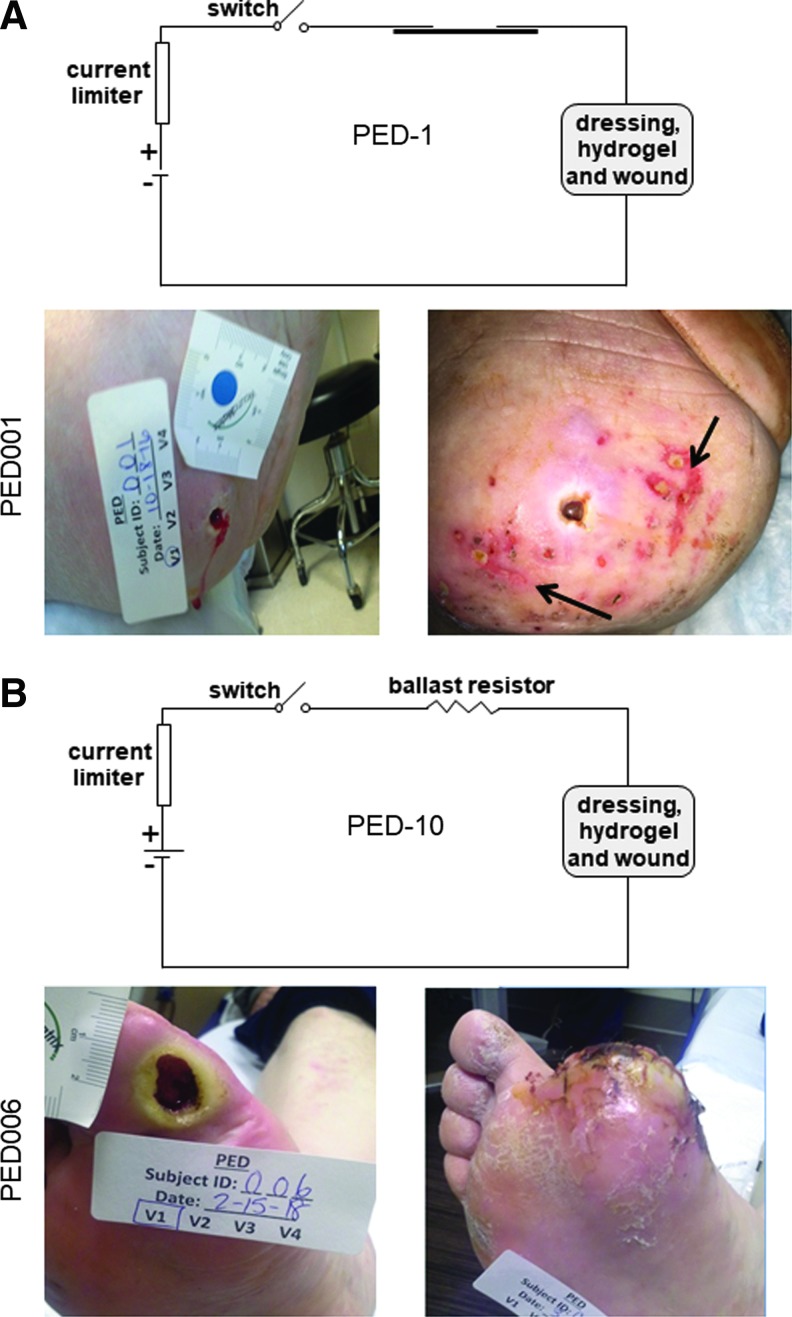
Figure 3.
Mild AEs associated with clinical PED-1 treatment application. (A) The first subject with PED-1 application reported AE associated with the PED treatment. This report described that during the first dressing change, the nurse noted a skin discoloration and minor irritation in skin surrounding the ulcer that was documented in the attached photograph (right). The treatment of PED-1 was immediately discontinued, and the event reported to IRB as unanticipated nonserious AE. At this stage, the study was put on a hold, and clinical PED was redesigned to replace 1 kΩ with 10 kΩ ballast resistor to reduce current to wounds and surrounding tissue. This PED was named as PED-10. Subsequently all subjects received PED-10. (B) Representative image of a wound treated with PED-10. AEs, adverse events; IRB, Institutional Review Board; PED-1, dressing with 1 kΩ ballast resistor.