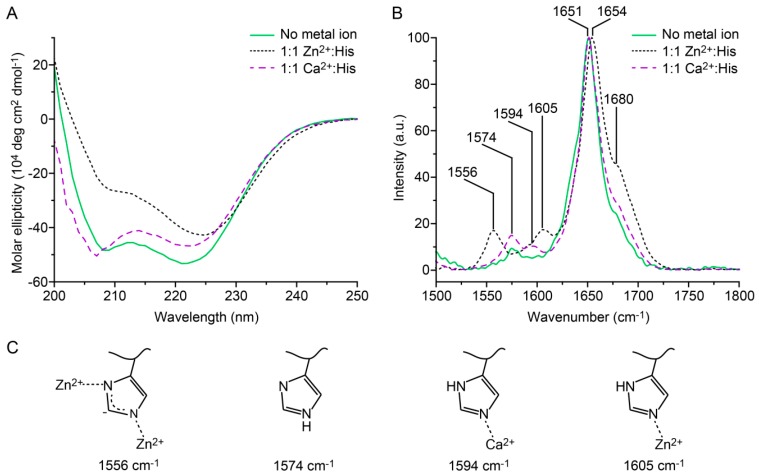
Figure 2.
Circular dichroism and Raman spectroscopy of A4H3B4H3. (A) Circular dichroism spectra measured in noncoordinating PIPPS buffer. (B) Raman spectroscopy performed on dried thin films of the coiled coil (CC). With both techniques, the CC was studied in the absence of metal ions and in the presence of 1:1 Zn2+:His or 1:1 Ca2+:His (negative control). (C) Scheme of the protonation and coordination states of His observed in Raman spectroscopy. The amide I peaks at 1651 cm−1 and 1654 cm−1 indicate an α-helical secondary structure. The assignment of the peak at 1680 cm−1 is less clear and can indicate the existence of β-sheet or unordered secondary structures [41,42,43].