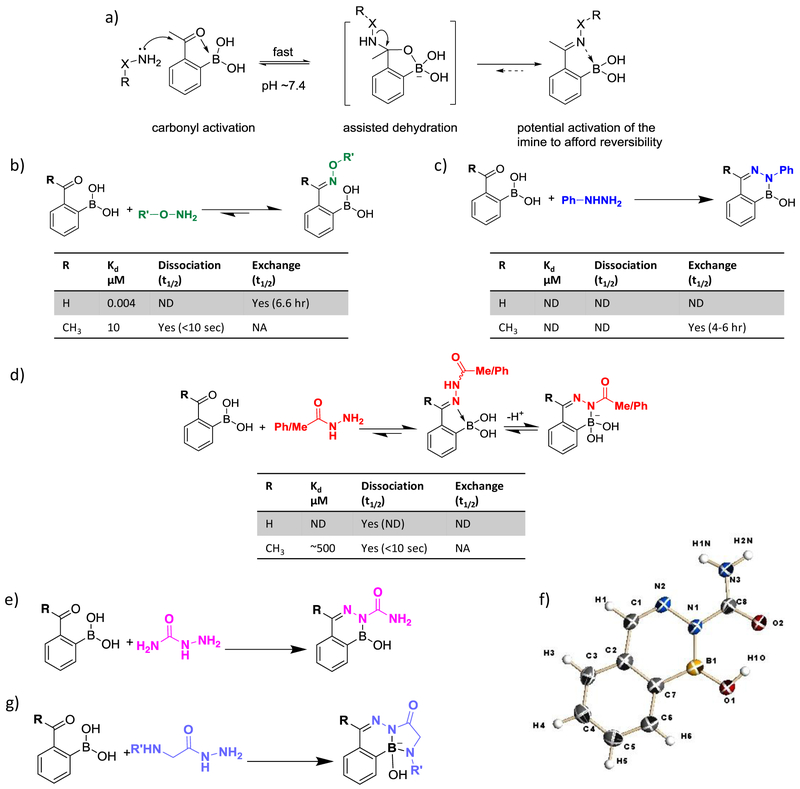
Figure 4.
Fast bioorthogonal conjugation of 2-FPBA/APBA with α-nucleophiles. (a) Mechanistic illustration of the boronic acid-accelerated conjugation of 2-FPBA/APBA with α-nucleophiles. (b) Oxime formation of 2-FPBA and 2-APBA yielding distinct product stability. ND: Not Determined; NA: Not Applicable. Exchange is considered Not Applicable to highly dynamic reactions. (c) Stable diazaborine formation of 2-FPBA/APBA upon conjugation with phenylhydrazine. (d) Dynamic conjugation of 2-FPBA/APBA to carbohydrazides. (e) Stable diazaborine formation of 2-FPBA/APBA with semicarbazide. (f) Crystal structure of a diazaborine conjugate of 2-FPBA and semicarbazide. (g) Improving the conjugate stability of carbohydrazide with an α-amino group that forges additional B-N bond.