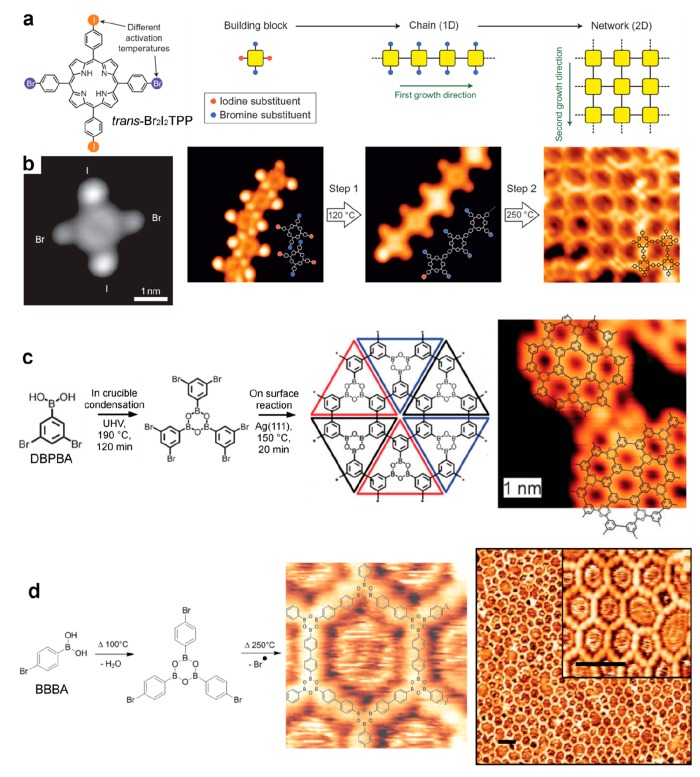
Figure 7.
Sequential coupling strategy. (a) Chemical structure of a porphyrin derivative (trans-Br2I2TPP) bearing both Br and I substituents in para positions, respectively. In a first step the I positions react to build 1D chains, and the Br positions react in a second step at a higher annealing temperature to connect the chains and build 2D networks. (b) Corresponding STM images showing the isolated precursors, supramolecular chains, covalent chains, and 2D covalent networks. (c) For a precursor bearing both bromine and boronic acid functions (DBPBA), the condensation of the boronic acid moieties leads in a first step to the formation of boroxine trimers that couple covalently at a higher annealing temperature in a second step. (d) Similar strategy with a para-bromide-boronic acid (BBBA). (a, b) Reprinted by permission from ref (145). Copyright 2012 Springer Nature. (c) Adapted with permission from ref (157). Copyright 2011 Royal Society of Chemistry. (d) Adapted with permission from ref (141). Copyright 2012 American Chemical Society.