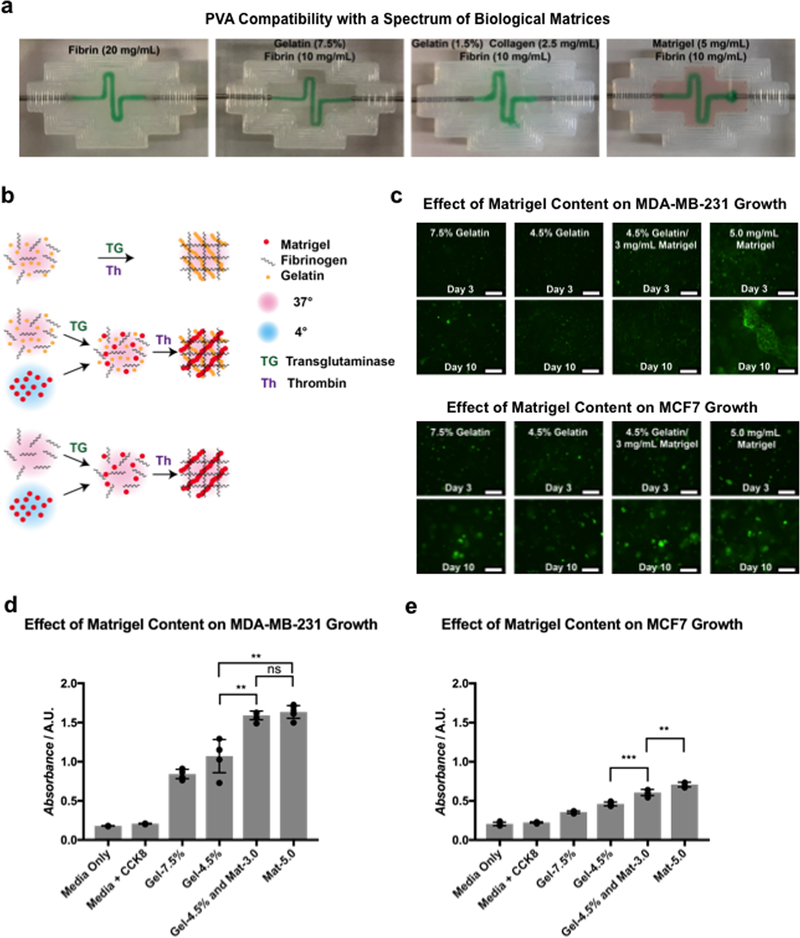
Figure 2.
Development of optimally cell-compatible constructs based on biologically derived matrix materials: a) Images indicating successful evacuation of PVA from matrices composed of various biological materials. b) Schematic representation of the materials-testing procedure. Gelatin/fibrin matrices were formulated by mixing both components with transglutaminase, then polymerizing with thrombin at 37 °C. Matrigel/fibrin and gelatin/Matrigel/fibrin blended matrices were formulated by mixing all components except Matrigel with transglutaminase at 37 °C, then adding Matrigel and polymerizing with thrombin. Matrigel was maintained at 4 °C, while all other components were maintained at 37 °C during the procedure. c) Fluorescent images showing growth of GFP-labeled MDA-MB-231 cells and MCF-7 cells in matrices of various compositions. Scale bars: 250 μm. d,e) Absorbance measurements of MDA-MB-231 cells and MCF-7 cells grown in matrices of various compositions, obtained using a CCK8 assay (n = 4 with P values **P < 0.01 and ***P < 0.001).